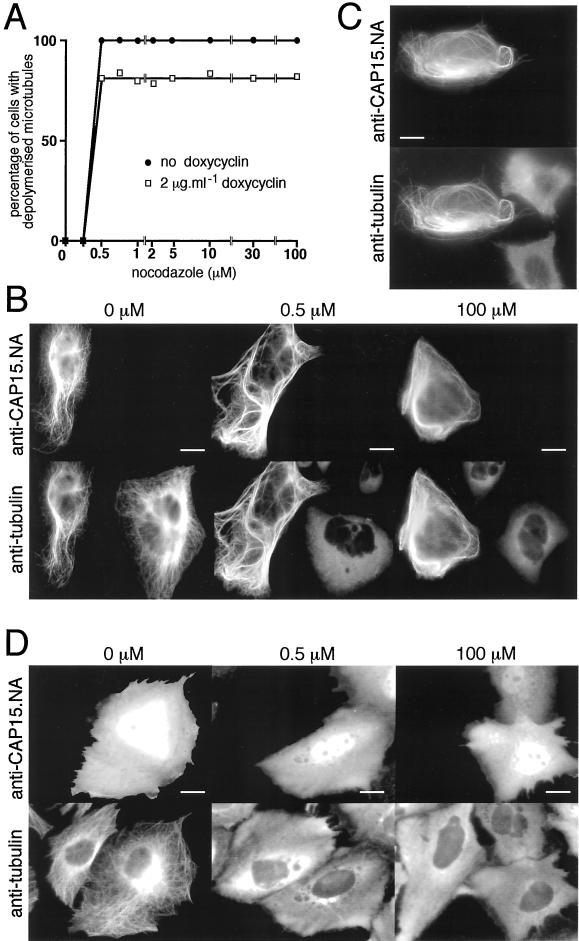
Figure 7.
Heterologous expression of CAP15 in CHO-K1 and HeLa cells. (A) Measurement of microtubule stability in CHO-K1 cells transfected by pTRE2-CAP15. The graph represents the percentage of cells presenting a complete depolymerization of the microtubule network as a function of nocodazole concentration. Doxycyclin-induced (▪) or uninduced (●) cells were treated for 30 min with various concentrations of nocodazole and analyzed by immunofluorescence to monitor the drug-induced microtubule depolymerization as shown in B. Approximately 20% of the doxycyclin-induced cells transfected by pTRE2-CAP15 were resistant to microtubule depolymerization after treatment with 0.5 μM nocodazole. (B) Immunofluorescence analysis of nocodazole-treated cells. Doxycyclin-induced cells were treated with 0, 0.5, or 100 μM nocodazole for 30 min and stained with anti-CAP15.NA serum and monoclonal anti-tubulin. Bar, 10 μm. (C) Cold-resistance of microtubules from HeLa cells transfected with pcDNA-CAP15. Transfected cells were treated 30 min at 4°C and stained with anti-CAP15. NA serum and monoclonal anti-tubulin. Bar = 10 μm. (D) Same experiment as in B, except that the CHO-K1 cells were transfected with the pcDNA3-CAP15nt plasmid, which encodes the CAP15 without the eight first amino acids. Bar, 10 μm. The first three panels show that the microtubule network of CHO-K1 (A and B) or HeLa (C) cells expressing CAP15 are resistant to nocodazole (A and B) and cold (C) treatments, while untransfected cells present a complete microtubule depolymerization under these conditions, indicating that CAP15 stabilizes microtubules. Panel D shows that expression in CHO-K1 cells of the truncated form of the protein does not bind to microtubules and does not protect microtubules against nocodazole treatment.