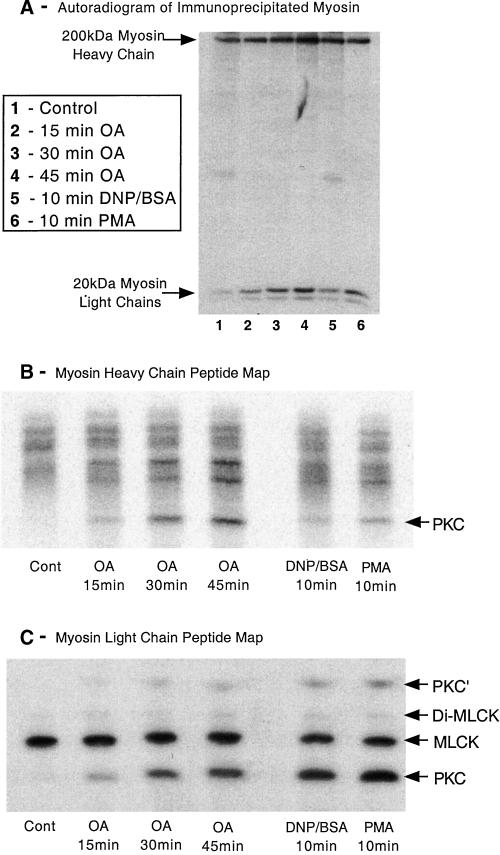
Figure 9.
Phosphopeptide mapping of myosin heavy and light chains. Adherent monolayers of RBL-2H3 cells that had been incubated for 2 h with 100 μCi/ml [32P]orthophosphoric acid were washed then incubated in a buffer containing 1 μM OA for 15, 30, or 45 min, or activated with antigen (100 ng/ml DNP-BSA) or PMA (50 nM) for 10 min. Cells were lysed and myosin immunoprecipitated using specific antibodies, before being separated on 5 or 12.5% SDS-PAGE and subjected to autoradiography as detailed in MATERIALS AND METHODS. (A) Autoradiogram of a 12.5% gel depicting the changes in phosphorylation of the myosin heavy and light chains. The myosin heavy and light chains were then excised from the gel, digested with trypsin, and the peptides separated on a one-dimensional IEF gel as detailed in MATERIALS AND METHODS. (B and C) Heavy chain and light chain peptide maps, respectively. Direction of the IEF on both peptide maps is toward the anode, which is at the top of B and C. The peptide labeled PKC in the MHC phosphopeptide map corresponds to the peptide in platelet myosin phosphorylated in vitro by PKC (Moussavi et al., 1993). The two MLC peptides that were phosphorylated by PKC (PKC and PKC′) correspond to Ser-1 or Ser-2 and are generated by different tryptic cleavage (Moussavi et al., 1993). MLCK forms peptides monophosphorylated at Ser-19 and diphosphorylated at Thr-18 and Ser-19. The amount of myosin immunoprecipitated was checked by Coomassie blue staining and confirmed there was little difference in loading between samples. The images shown are representative of four separate experiments.