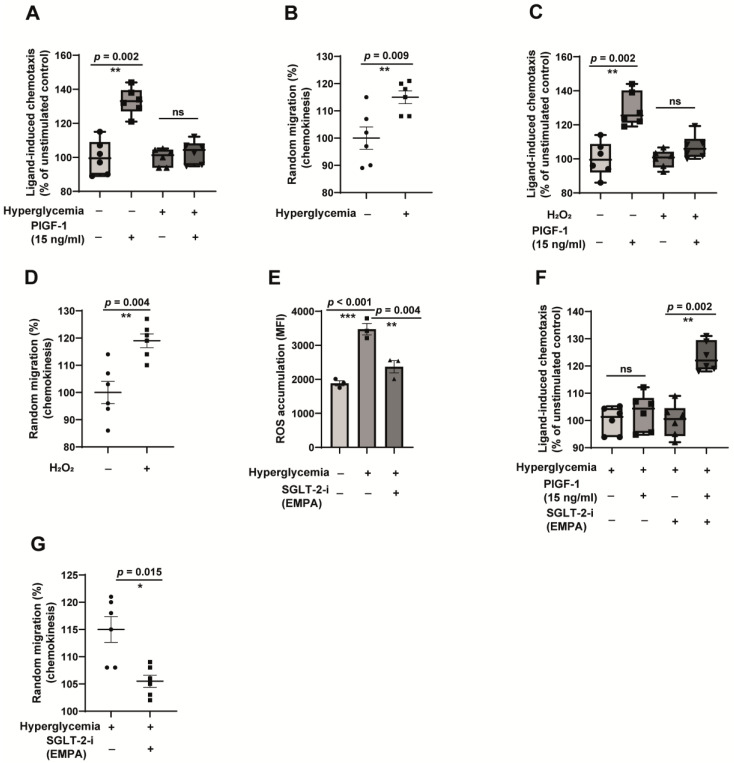
Figure 4.
Hyperglycaemia-induced monocyte dysfunction is reversed by empagliflozin through the modulation of oxidative stress. (A) CD14++CD16− monocytes were cultured in vitro in normoglycemic (5 mM glucose) or hyperglycaemic (30 mM glucose + 100 µM methylglyoxal) for 48 h and were analysed for their ability to undergo chemotaxis (directional migration) towards arteriogenic stimuli PlGF-1. Boyden chamber assays were performed. n = 6. (B) CD14++CD16− monocytes were cultured in vitro in normoglycemic (5 mM glucose) or hyperglycaemic (30 mM glucose + 100 µM methylglyoxal) for 48 h and were analysed for their ability to undergo chemokinesis (random migration). Checkerboard analyses were performed for this. n = 6. All data are means ± SEM. (C,D) CD14++CD16− monocytes were cultured in vitro in normoglycemic (5 mM glucose) conditions in the presence of 200 µM H2O2 for 24 h. After that, the cells were analysed for their ability to undergo chemotaxis towards PlGF-1 and chemokinesis using Boyden chamber assays. n = 6. All data are means ± SEM. (E) CD14++CD16− monocytes were cultured in vitro in normoglycemic (5 mM glucose) or hyperglycaemic (30 mM glucose + 100 µM methylglyoxal) for 48 h. The reactive oxygen species (ROS) accumulated was detected by fluorescence spectroscopy using 5-(and-6)-carboxy-2′,7′-difluorodihydrofluorescein diacetate (H2-DFFDA) reagent. n = 3. All data are means ± SEM. (F,G) CD14++CD16− monocytes were cultured in vitro under hyperglycaemic (30 mM glucose + 100 µM methylglyoxal) conditions for 48 h in the presence or absence of 100 ng/mL SGLT-2 inhibitor empagliflozin. After that, the cells were analysed for their ability to undergo chemotaxis towards PlGF-1 and chemokinesis using Boyden chamber assays. n = 6. All data are means ± SEM. ns = non-significant. * p < 0.05, ** p < 0.01 and *** p < 0.001.