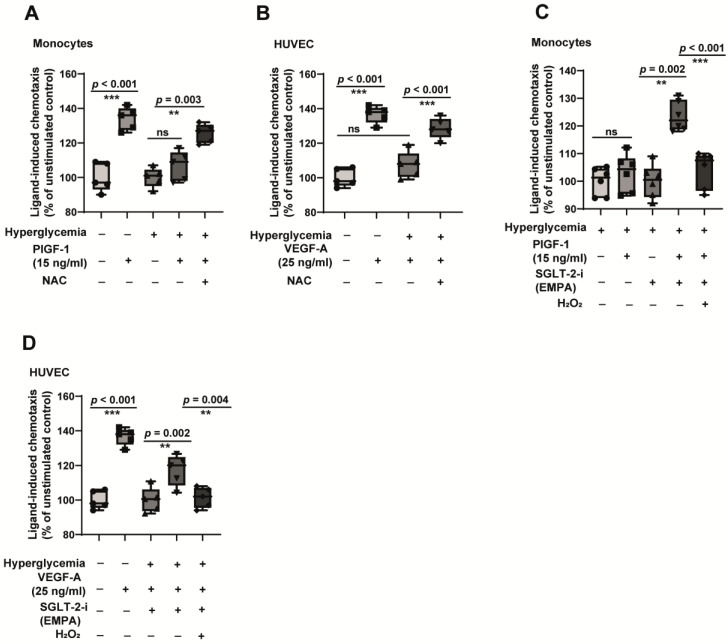
Figure 6.
Manipulating the redox status of the cells recapitulates the dysfunction phenotype. (A) CD14++CD16− monocytes were cultured in vitro in normoglycemic (5 mM glucose) or hyperglycaemic (30 mM glucose + 100 µM methylglyoxal) for 48 h in the presence or absence of 5 mM N-acetylcysteine (NAC) and were analysed for their ability to undergo chemotaxis (directional migration) towards arteriogenic stimuli PlGF-1. Boyden chamber assays were performed. n = 5. (B) HUVECs were exposed to in vitro normoglycemic conditions (5 mM glucose) or hyperglycaemic conditions mimicking a diabetic milieu (30 mM Glucose + 100 µM methylglyoxal) for 24 h in the presence or absence of 5 mM N-acetylcysteine (NAC). The cells were then analysed for their ability to undergo chemotaxis (directional migration) towards angiogenic stimuli VEGF-A. Boyden chamber assays were performed. n = 5. (C) CD14++CD16− monocytes were cultured in vitro under hyperglycaemic (30 mM glucose + 100 µM methylglyoxal) conditions for 48 h in the presence or absence of 100 ng/mL SGLT-2 inhibitor empagliflozin. In addition, cells were treated with or without 200 µM H2O2. After that, the cells were analysed for their ability to undergo chemotaxis towards PlGF-1 in the presence of 200 µM H2O2. n = 5. (D) HUVECs were exposed to in vitro normoglycemic conditions (5 mM glucose) or hyperglycaemic conditions mimicking a diabetic milieu (30 mM Glucose + 100 µM methylglyoxal) for 24 h in the presence or absence of 100 ng/mL SGLT-2 inhibitor empagliflozin. In addition, cells were treated with or without 200 µM H2O2. After that, the cells were analysed for their ability to undergo chemotaxis towards VEGF-A using Boyden chamber assays in the presence of 200 µM H2O2. n = 5. ns = non-significant. ** p < 0.01 and *** p < 0.001.