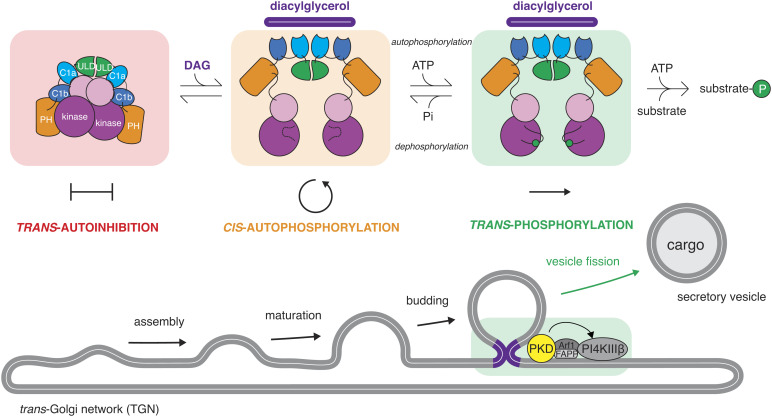
Fig. 7.
PKD autoinhibition in trans regulates activation loop autophosphorylation in cis. Model for the control of PKD by DAG binding and subsequent triggering of cargo vesicle fission from the TGN. PKD adopts a constitutively dimeric conformation in the cytosol of cells in which its kinase domains are maintained in a trans-autoinhibitory face-to-face dimer. Binding to DAG at the TGN, presumably in the vicinity of the bud neck of cargo vesicles, results in conformational changes that relieve the inhibitory dimerization of its kinase domains, leading to activation loop cis-autophosphorylation. Activation loop phosphorylation both promotes trans-phosphorylation of substrates and prevents reassembly of the kinase domains.