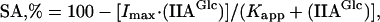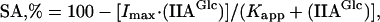Effect of the T-477-N substitution on IIA
Glc inhibition of glycerol kinase. Initial velocities of glycerol kinase catalyzed reactions were determined at pH 7.0 and 25°C as described in Materials and Methods. The points show the data that were obtained for the normal or T-477-N glycerol kinase and the lines show the fits of the data to the following equation:
where SA is specific activity,
Imax is the maximum extent of inhibition and
Kapp is the apparent dissociation constant for IIA
Glc binding to glycerol kinase. The fits yield the following parameters for normal glycerol kinase:
Imax, 96 ± 1%;
Kapp, 0.95 ± 0.06 μM. Those for T-477-N glycerol kinase are as follows:
Imax, 96% (fixed);
Kapp, 370 ± 70 μM. Conditions: 2 mM glycerol, 2.5 mM ATP, 0.1 mM ZnCl
2, and IIA
Glc as indicated on the
x axis. Enzyme concentration, 0.5 μg/ml. WT, wild type.