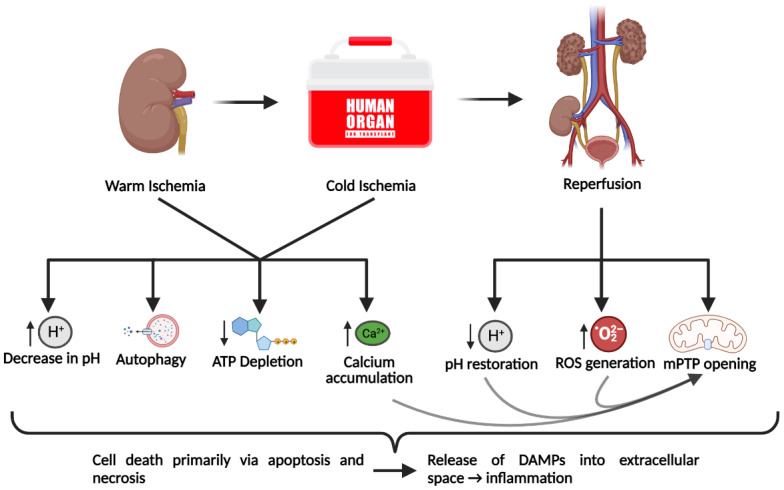
Figure 1.
Cellular mechanisms of ischemia-reperfusion injury incurred through solid organ transplantation. The warm ischemia period begins with the interruption of perfusion to the donor organ and continues until the organ is flushed with hypothermic preservation solution, which marks the beginning of cold ischemia. The cold ischemic period typically consists of 4 °C cold storage of the procured organ and continues until the graft is implanted into the recipient. Together, these two ischemic periods lead to the generation of a pathological state that is included in the depletion of ATP due to the unavailability of oxygen, calcium accumulation, and decrease in cellular pH due to altered ion channel activity, and autophagy, which likely occurs to provide a source of energy. Subsequent reperfusion of the transplanted organ induces a paradoxical response whereby the injury is exacerbated. The restoration of blood flow rapidly restores pH levels and leads to the massive generation of reactive oxygen species (ROS), which together with the high intracellular calcium concentration can induce the opening of mitochondrial permeability transition pores (mPTP). Collectively, these effects can induce cell death, primarily via apoptosis and necrosis. Necrotic cell death releases danger-associated molecular patterns (DAMPs) into the extracellular space and leads to an inflammatory response. Figure created with BioRender.com.