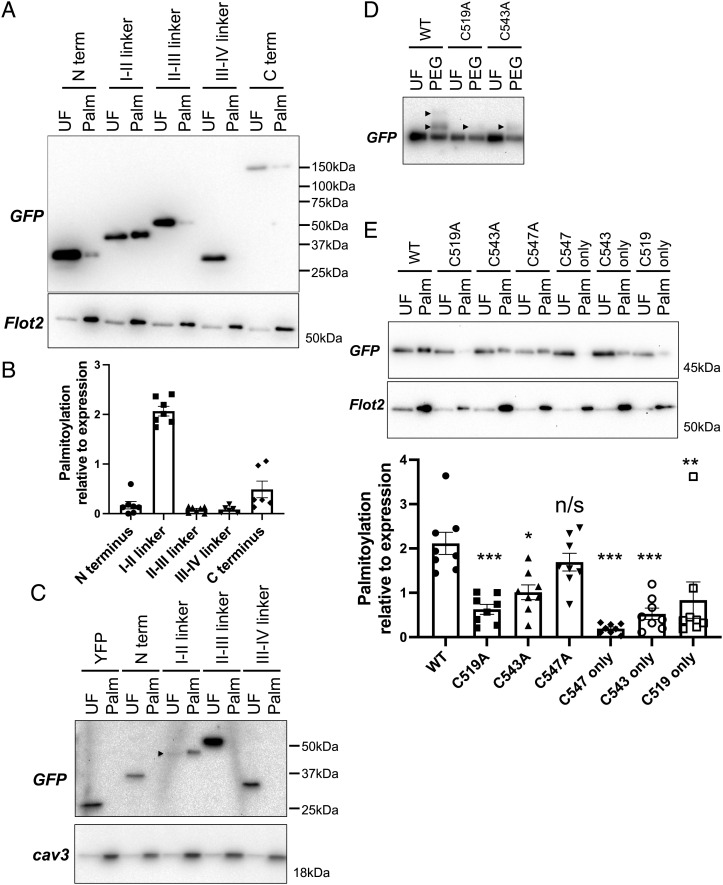
Fig. 3.
Palmitoylation of the I–II linker of α1C in transfected HEK cells. (A) YFP fusion proteins of the α1C intracellular regions were expressed in HEK cells, and palmitoylated proteins (Palm) were then purified and immunoblotted alongside unfractionated (UF) cell lysates. The palmitoylated protein flotillin 2 is a positive control for the acyl-RAC reaction. (B) Palmitoylation of YFP fusion proteins normalized to expression in HEK cells (N = 9). (C) The I–II linker (but no other intracellular regions) is also palmitoylated in the cardiomyoblast H9C2 line. For clarity, the I–II linker is marked with an arrowhead. Blots are representative of two independent experiments. (D) PEGylation assay identifies dual palmitoylation of the I–II linker. In this assay, palmitates are replaced with a 5-kDa PEG molecule, which causes a band shift on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (arrowhead), according to the number of palmitoylation sites. Mutation of either C519 or C543 removes one palmitoylation site. (E) Identification of the palmitoylated cysteines in a YFP fusion of α1C I–II linker. Single and double cysteine-to-alanine mutants were expressed in HEK cells, and palmitoylated proteins prepared by resin-assisted capture (WT, wild type; “only” refers to all cysteines, but this one removed). Acyl-RAC captures proteins regardless of whether they are singly or doubly palmitoylated. Capture of each mutant (relative to expression) is shown in the bar chart below the representative blot (error bars represent SEM). Mutation of a single cysteine does not abolish palmitoylation, but mutation of cysteines in positions 519 and 543 does (UF: unfractionated cell lysate, Palm: palmitoylated proteins, N = 8). Statistical comparisons were made using ANOVA followed by Dunnett’s multiple comparisons test and are to WT. ***P < 0.001, **P < 0.005, *P < 0.05, n/s, not significant.