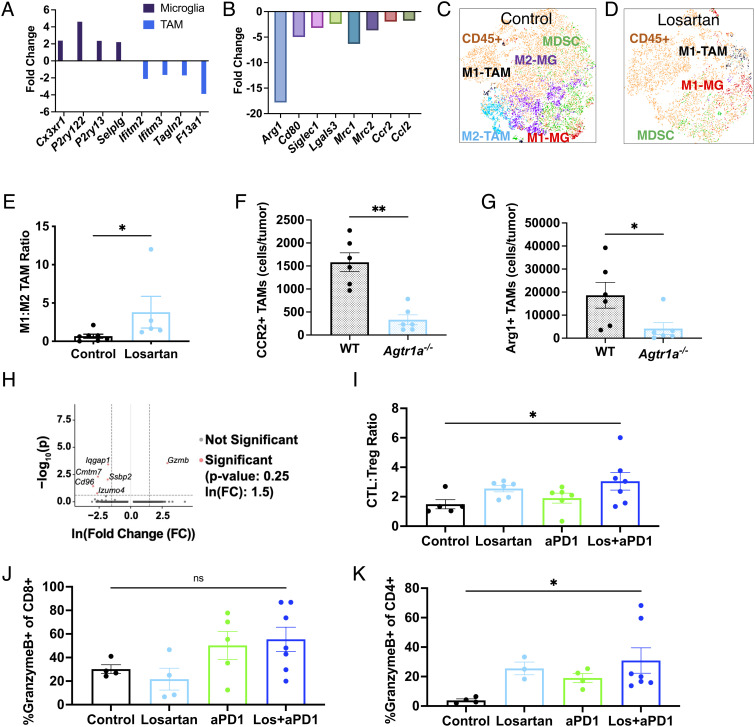
Fig. 4.
Losartan promotes antitumor immunity in the GBM TME. Applying the human-derived signatures from our previous work (16), losartan is found to enrich microglia-like signatures and downregulate global (A) and M2-like (B) TAM signatures vs. controls as assessed in bulk RNASeq samples from GL261 (n = 3). t-distributed stochastic neighbor embedding (t-SNE) plots of flow cytometry data of myeloid populations reveal (C) a diverse and largely immunosuppressive (“M2”) microenvironment in GL261 controls that is (D) reprogrammed by losartan treatment to feature fewer myeloid cells that are polarized for anti-tumor (“M1”) activity (MG, microglia). (E) Losartan increases the ratio of anti- to pro-tumor TAMs, assessed via flow cytometry (n = 5 to 7). Highly suppressive TAM subsets (F) CCR2+ and (G) Arg1+ (of CD45hiCD11b+F4/80+) are downregulated in GL261 tumors implanted in Agtr1a−/− mice compared to those implanted in wild-type C57Bl/6 mice. (H) scRNASeq of CD8+ T cells reveals heightened Gzmb expression under combined treatment compared to anti-PD1 monotherapy. Losartan+anti-PD1 treatment increases (I) cytotoxic (CTL; CD45+CD3+CD8+GranzymeB+) to regulatory (Treg; CD45+CD3+CD4+FoxP3+) T cell ratios in the tumor, and effector Granzyme+ CD8 (J, not significant) and CD4 (K) T cells in the cervical lymph nodes. (Sequencing: all FDR q-values < 0.25, FC > |2|, adjusted P values < 0.05. Flow cytometry: Mann–Whitney unpaired t test or one-way ANOVA with Tukey’s post hoc test; *P < 0.05.)