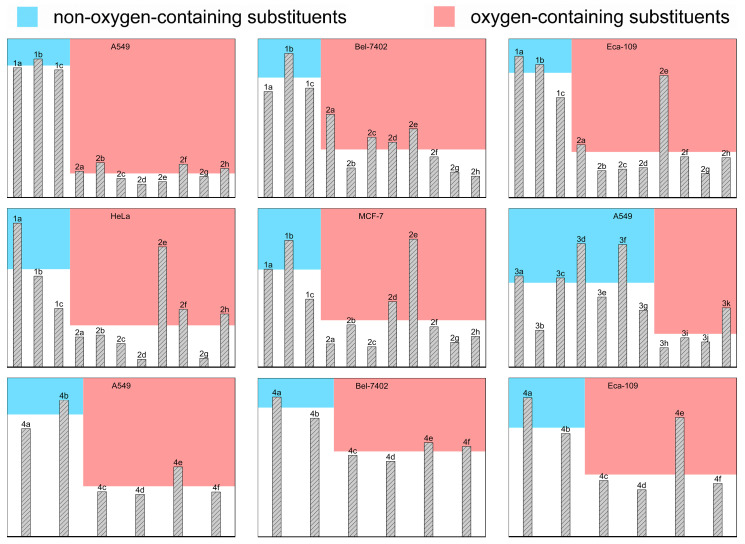
Figure 7.
The in vitro anticancer activity of substituted 2,2′:6′,2″-terpyridine(tpy) complexes 1a–1c, 2a–2h, 3a–3k [65] and 4a–4f [44] against different cancer cell lines, including A549, Bel-7402, Eca-109, HeLa and MCF-7. The heights of the blue and red blocks on the background represent the average performance of the anticancer activity of the non-oxygen-containing substituents and oxygen-containing substituent-modified complexes, respectively. Here, 3a = [Cu(tpy)Cl2], 3b = [Cu(4′-(4′-cyano-phenyl)-tpy)Cl2], 3c = [Cu(4′-(4′-iodo-phenyl)-tpy)Cl2], 3d = [Cu(4′-(4′-bromo-phenyl)-tpy)Cl2], 3e = [Cu(4′-(4′-chloro-phenyl)-tpy)Cl2], 3f = [Cu(4′-(4′-fluoro-phenyl)-tpy)Cl2], 3g = [Cu(4′-(p-hydroxyl-phenyl)-tpy)Cl2], 3h = [Cu(4′-(m-hydroxyl-phenyl)-tpy)Cl2], 3i = [Cu(4′-(o-hydroxyl-phenyl)-tpy)Cl2], 3j = [Cu(4′-(methoxyl-phenyl)-tpy)Cl2], 3k = [Cu(4′-(4′-methylsulfonyl-phenyl)-tpy)Cl2], 4a = [Zn(4′-(4′-methyl-phenyl)-tpy)Br2], 4b = [Zn(4′-(4′-methyl-phenyl)-tpy)I2], 4c = [Zn(4′-(4′-methylsulfonyl-phenyl)-tpy)Br2], 4d = [Zn(4′-(4′-methylsulfonyl-phenyl)-tpy)I2], 4e = [Zn(4′-(4′-methoxyl-phenyl)-tpy)Br2], 4f = [Zn(4′-(4′-methoxyl-phenyl)-tpy)I2].