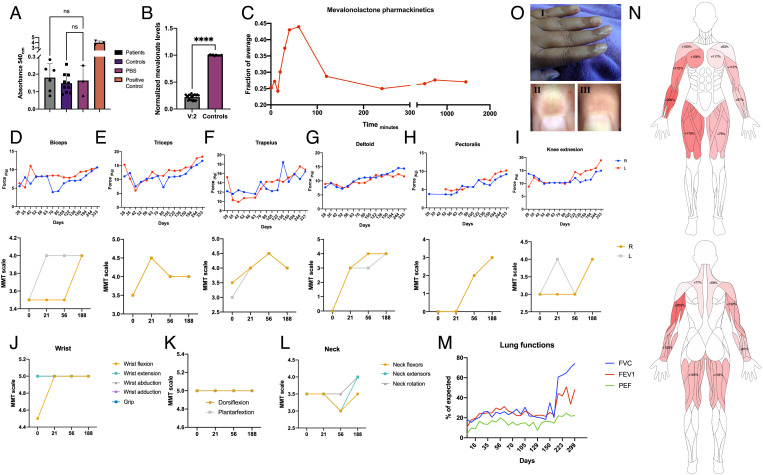
Fig. 4.
Oral mevalonolactone treatment in human HMGCR-LGMD. (A) Anti-HMGCR autoantibodies titer in patients (n = 6) and healthy controls (n = 10). (B) Plasma mevalonolactone levels of patients V:2 (n = 20 on multiple occasions) and healthy controls (n = 10), normalized to average level of controls. (C) Mevalonolactone levels in peripheral blood of V:2 after an oral dose of 16 mg/kg mevalonolactone, normalized to average level of controls. (D–I) Evaluation of muscle strength of patient V:2 throughout the treatment period by dynamometry (Top) and manual muscle test (MMT; Bottom) by an experienced neurologist. (J–L) Evaluation of distal muscles throughout the treatment by an experienced neurologist. (M) Lung functions throughout the treatment period assessed by spirometry. (N) Muscle strength improvement "heat-map" by dynamometry, precent improvement from weakest state. (O) Pigmentation in the proximal nail fold which appeared occasionally following treatment. I: Gross appearance; II: Dermatoscopic image of the index finger showing pigmentation; and III: Dermatoscopic image of the same index finger several days later, pigmentation has resolved. Statistical analysis using t-test.