Fig. 2.
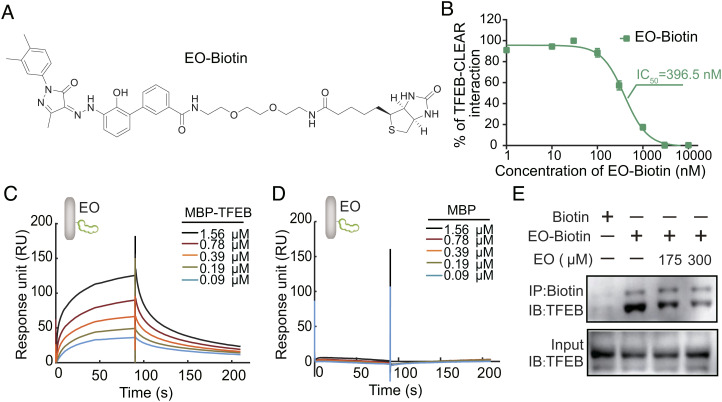
Binding affinity between EO and TFEB was quantified by a biotinylated EO. (A) Chemical structure of biotinylated EO (EO-Biotin). (B) Serial diluted (0 to 10 μM) EO-Biotin was titrated to a solution containing 50 nM TFEB and 5 nM FAM-CLEAR DNA element. EO-Biotin inhibited the TFEB-CLEAR DNA interaction with an IC50 of 396.5 nM. Error bars represent the SEMs of four repeats. (C) EO-Biotin was immobilized on a SA chip. The affinity between the bHLH-LZ domain of TFEB and EO was measured by SPR experiment. MBP-TFEB bHLH-LZ interacts with EO with kon = 4.59E4 ± 5.60E2 (M s)−1, koff = 1.58E−2 ± 1.40E−4 s−1, and Kd = 345.7 nM. (D) The interaction between MBP control and EO cannot be detected in the SPR experiment. (E) Endogenous TFEB from the U87 cell lysate could be co-precipitated with EO-Biotin, while the competition of unlabeled EO reduced the co-precipitated TFEB. Abbreviations: IB, immunoblot; IP, immunoprecipitation.