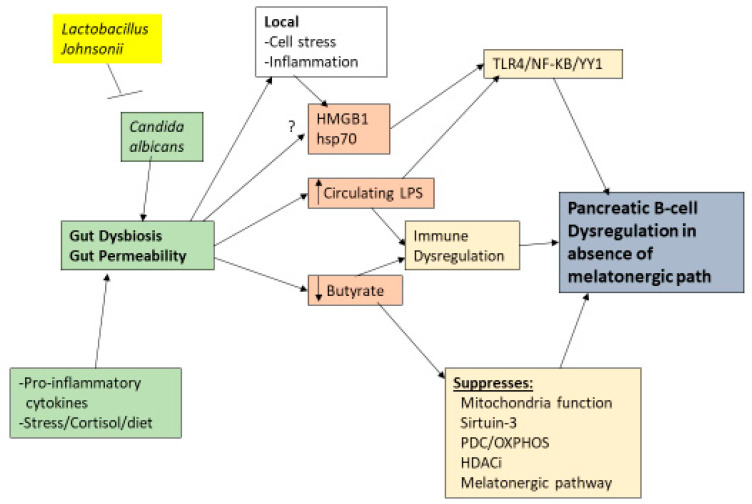
Figure 3.
Shows the role of gut dysbiosis and gut permeability in T1DM. Candida albicans fungal infection in the gut can lead to gut dysbiosis and associated gut permeability, as can heightened pro-inflammatory cytokines, stress/cortisol, and many dietary factors. Lactobacillus johnsonii bacteria can eliminate Candida albicans from the gut. Gut permeability increases circulating LPS, which can dysregulate the immune response via TLR4 activation, leading to pro-inflammatory transcription factors, NF-κB and YY1, in immune cells and pancreatic B-cells. Butyrate suppression attenuates its optimization of mitochondrial function via increased sirtuin-3 and PDC induction that enhances OXPHOS, at least partly via the melatonergic pathway. Suppressed butyrate will also attenuate its capacity as a HDACi, leading to altered epigenetic regulation, with consequences for local cellular stress and inflammation, which increases HMGB1 and hsp70. Both of these TLR4 ligands may also be released by the gut, including in exosomes. These gut-derived changes will have direct impacts on pancreatic B-cells and other pancreatic islet cells as well as indirect effects via alterations in patterned immune responses. Damage in pancreatic B-cells will be at least partly dependent upon the suppression of the melatonergic pathway. Abbreviations: HDACi: histone deacetylase inhibitor; HMGB1: high-mobility group box 1; hsp70: heat shock protein 70; LPS: lipopolysaccharide; OXPHOS: oxidative phosphorylation; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; PDC: pyruvate dehydrogenase complex.