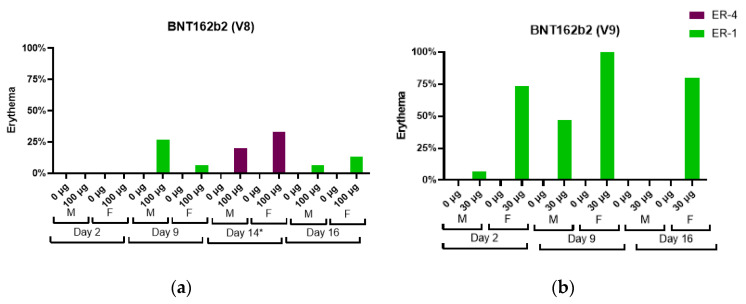
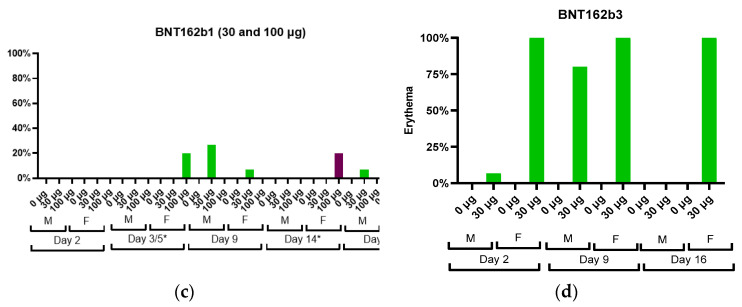
Figure 3.
Erythema incidence and severity in male (M) and female (F) Wistar Han rats immunized intramuscularly (IM) with (a) BNT162b2 V8 (100 µg), (b) BNT162b2 V9 (30 µg), (c) BNT162b3 (30 µg), and (d) BNT162b1 (30 µg or 100 µg) or buffer control. Erythema measurements from Dosing Phase Days 2, 3/5 *, 9, 14 *, and 16. * Days 3/5 and 14 are only available for Study 1.