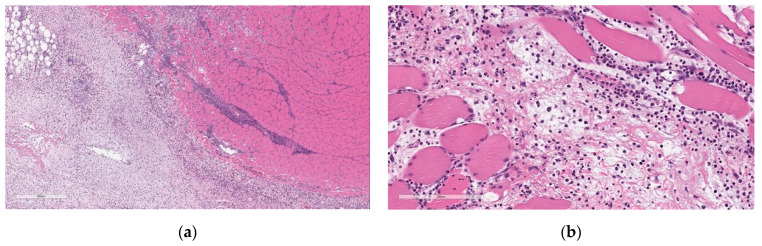
Figure 5.
Histopathologic features of Injection site inflammation and edema at the end of the dosing phase. (a) Injection site sections of BNT162b2 (V9) treated animals euthanized 2 days after the third dose (Day 17) showed inflammatory cells admixed with abundant pale eosinophilic fluid (edema) infiltrating and expanding subcutaneous tissue and connective tissue of skeletal muscle. (b) Higher magnification of (a) showing.