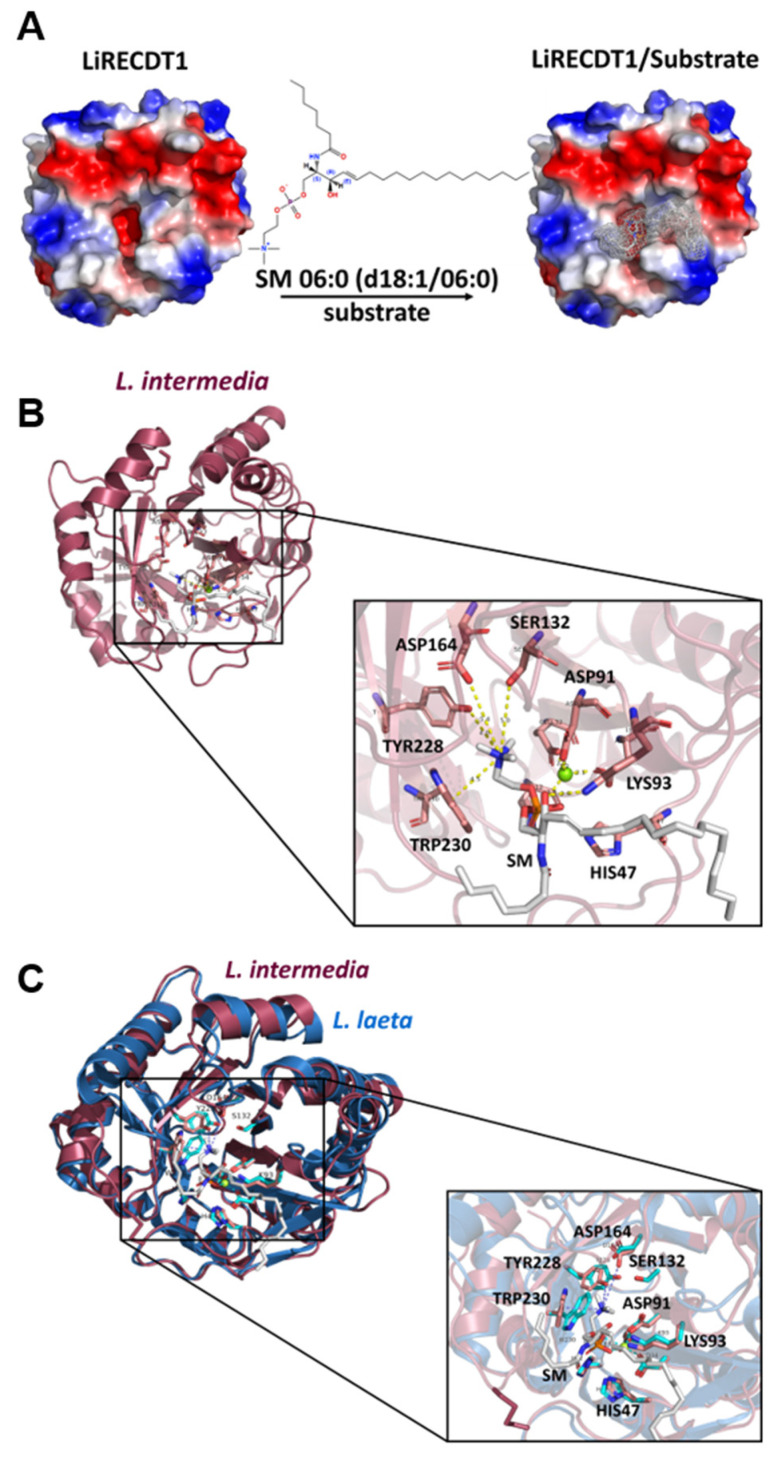
Figure 6.
Structural models of brown spider phospholipase-D with sphingomyelin—SM 06:0 (d18:1/06:0). (A) Electrostatic view of LiRecDT1 indicating the position of sphingomyelin in the catalytic site of the enzyme. In blue, positive regions, red negative regions and white the hydrophobic regions. (B) Cartoon view of LiRecDT1 (red) bound to sphingomyelin (gray), depicting the residues important for interaction with the substrate and for catalysis. (C) Superposition of L. intermedia PLD (PDB 3RLH) with L. laeta Smase I (PDB 1XX1) demonstrating a high level of structural homology and sequence conservation within active site clefts. L. intermedia PLD has depicted as red cartoon with disulfide tethered catalytic and flexible loops highlighted in magenta. L. laeta SmaseI is rendered as blue cartoon with catalytic and flexible loops highlighted in cyan. Invariant active site residues of L. intermedia and L. laeta enzymes are displayed as sticks magenta and cyan with L. intermedia PLD numbering. The docked pose of SM 06:0 (d18:1/06:0) substrate is shown as gray sticks. Images were prepared using Pymol [29]. For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.