Abstract
COVID-19 patients occasionally present with diarrhoea. Our objective was to estimate the risk of developing the severe disease in COVID-19 patients with and without diarrhoea and to provide a more precise estimate of the prevalence of COVID-19-associated digestive symptoms. A total of 88 studies (n = 67,794) on patients with a COVID-19 infection published between 1 January 2020 and 20 October 2022 were included in this meta-analysis. The overall prevalence of digestive symptoms was 27% (95% confidence interval (CI): 21–34%; I2 = 99%). According to our data, the pooled prevalence of diarrhoea symptoms in the 88 studies analysed was 17% (95% CI: 14–20%; I2 = 98%). The pooled estimate of nausea or vomiting in a total of 60 studies was 12% (95% CI: 8–15%; I2 = 98%). We also analysed 23 studies with eligible individuals (n = 3800) to assess the association between the disease severity and diarrhoea. Individuals who had diarrhoea were more likely to have experienced severe COVID-19 (odds ratio: 1.71; 95% CI: 1.31–2.24; p < 0.0001; I2 = 10%). Gastrointestinal symptoms and diarrhoea are frequently presenting COVID-19 manifestations that physicians should be aware of.
Keywords: nCoV COVID-19, gastrointestinal tract, diarrhoea, SARS-CoV-2, nausea, vomiting, abdominal pain, anorexia
1. Introduction
Coronavirus disease 2019 (COVID-19) is the fifth pandemic after 1918 H1N1 (Spanish flu), 1957 H2N2 (Asian flu), 1968 H3N2 (Hong Kong flu) and 2009 H1N1 (Influenza) [1]. The disease was discovered in China, and its most common clinical symptoms are fever, cough, sore throat, rhinorrhoea, headache, fatigue, shortness of breath, abdominal pain and anosmia [2]. However, a small percentage of patients experience gastrointestinal symptoms, with diarrhoea being the most common symptom and thought to be present in 10–50% of the infected population [3,4]. The gastrointestinal tract is believed to be affected because of a direct viral invasion mediated by the binding of the virus to the angiotensin-converting enzyme-2 (ACE-2) receptor, thereby causing cytotoxic damage [5].
Emerging data indicate that diarrhoea is associated with severe COVID-19 and suggest that diarrhoea could be a reliable indicator of the onset of severe COVID-19 [6]. In other studies, diarrhoea has been linked to an increase in the severity of the COVID-19-associated pathology [7]. Although clinical research comparing COVID-19 in the presence and absence of diarrhoea symptoms has already been conducted, significant conclusive evidence has yet to surface, and most studies have been conducted on a small sample size.
Herein, we attempted to better understand the relationship between COVID-19 and gastrointestinal symptoms by comparing the risk of developing the severe disease in COVID-19 patients with diarrhoea, the most common gastrointestinal manifestation. We analysed various data points related to disease severity with diarrhoea and other gastrointestinal manifestations in COVID-19 patients by using a systematic review and a meta-analysis of previously published studies in order to generalise the correlation across different settings. According to numerous studies, patients with severe COVID-19 are more likely to develop diarrhoea than are patients with non-severe COVID-19. However, because of inconsistencies in the analysed results and a lack of data, the exact effect of diarrhoea on COVID-19 remains unknown. As a result, additional research on the prevalence of gastrointestinal manifestations and the diarrhoea-associated severity in COVID-19 patients is required.
2. Materials and Methods
On 7 June 2021, our protocol was registered with PROSPERO, the International Prospective Register of Systematic Reviews (registration number: CRD42021234776). The criteria for the studies’ inclusion and exclusion are listed in Table 1.
Table 1.
Table outlining the criteria applied for the herein undertaken study inclusion and exclusion.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Studies that included case-controls, cohorts, cross-sectional and prospective studies | Children, adolescents and pregnant women |
| Literature search on electronic databases, namely PubMed, PubMed Central, Embase, Scopus, Cochrane library and ProQuest (from 1 January 2020 and last updated on 20 October 2022) by using search terms that included ‘SARCOV-2′, ‘COVID-19′, ‘gastrointestinal symptoms’, ‘diarrhoea’ and ‘nCoV’ | Articles that did not provide information on gastrointestinal symptoms |
| Articles reporting gastrointestinal symptoms associated with the COVID-19 infection that included diarrhoea, nausea, vomiting, abdominal pain and loss of appetite | Case reports, preprints, no full-text articles and no availability of the diarrhoea-associated data |
| Studies comparing severe versus non-severe diarrhoea | No relevant data |
| A patient who met the definition of severe disease as follows: (i) dyspnoea present, (ii) a respiratory rate: 30 or >breaths per minute, (iii) blood oxygen saturation: 93% or less, (iv) ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2:FiO2): <300 mm Hg, (v) infiltrates in more than 50% of lung field, (vi) patient under mechanical ventilation and ICU (intensive care unit) admitted, (vii) APACHE II and SOFA scores higher for critically ill patients with COVID-19 infection, (viii) hyponatraemia or hypokalaemia |
2.1. Methodology of Search and Selection Criteria
A systematic review and a meta-analysis were performed to assess the severity of the COVID-19 infection in patients with diarrhoea versus those without diarrhoea, by comparing severely and non-severely ill patient groups. We searched reputed databases for articles published between 1 January 2020 and 20 October 2022 by using the medical subject heading and the keywords ‘nCoV’, ‘SARS-CoV-2′, ‘digestive system’, ‘diarrhoea’, ‘abdominal pain’, ‘nausea’, ‘vomiting’, ‘anorexia’, ‘favipiravir’, ‘lopinavir/ritonavir’, ‘antibodies’, ‘monoclonal’, ‘molnupiravir’, ‘Sequential Organ Failure Assessment (SOFA)’, ‘APACHE II’, ‘hyponatraemia’, ‘hypokalaemia’, ‘intensive care unit (ICU)’, ‘SARS-CoV-2 variants’ and ‘COVID-19 vaccines’.
We searched PubMed, Scopus, Embase, Cochrane Library, ProQuest and the WHO publications’ database. A secondary search was undertaken by using published study references. References from all studies were checked for any additional sources of knowledge. Only peer-reviewed, English-language studies were included in our search, and only those studies that had been accepted for publication were considered. The online Rayyan Systematic Review platform was used to manage all relevant articles, including duplicates. We included studies that reported the severity of diarrhoea in infected patients and excluded studies with no availability of diarrhoea data, no full-text articles, duplicate publications, review articles, studies with an identical population, case series and case reports.
2.2. Extraction of Data and Definitions
Two independent reviewers (SD and KP) screened the titles and abstracts that met the eligibility criteria. We obtained the full-text articles of the studies that passed our initial screening of titles and abstracts. Subsequently, the full-text of the remaining articles were reviewed to see if these articles satisfied the inclusion criteria and were appropriate for further analysis. When dissonance between reviewers occurred, the complete text and extracted data were further reviewed by a third reviewer (BH) to corroborate validity. Data extraction was performed by employing a pre-defined form that included the following data: author, date and year of publication, study design (cohort, cross-sectional, case–control, prospective and case series studies), country, sample size, patient demographics, vital signs, both participants with severe and those with non-severe diarrhoea, the prevalence of digestive manifestations (e.g., diarrhoea, abdominal pain, nausea, vomiting and loss of appetite), associated comorbidities (e.g., hypertension, cardiovascular disease, diabetes and neurological disease), Acute Physiology and Chronic Health Evaluation II (APACHE II) score, duration of ICU stay, SOFA score, hypokalaemia, hyponatraemia, COVID-19 variant associations, vaccine administration and use of antibiotics and antiviral treatments.
Severe COVID-19 infection was defined as the onset of more severe signs and symptoms 1 week after the onset of the first symptoms, an ICU admission for mechanical ventilation as required for patients with a respiratory rate of ≥30 breaths per minute and an oxygen saturation (SpO2) ≤93% and for patients with lung infiltrates of ≥50% resulting in dyspnoea, a ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) of <300 mm Hg [8], higher APACHE II and SOFA scores, an electrolyte imbalance (manifesting as hyponatraemia and hypokalaemia) and the use of antiviral drugs (e.g., lopinavir/ritonavir) causing diarrhoeal side effects. This study followed the recommendations outlined by the PRISMA guidelines.
2.3. Assessment of the Risk of Bias
Each study’s quality was determined by using inclusion and exclusion criteria and through grading (as good, fair or poor) by assigning stars to each domain according to the Newcastle–Ottawa Scale (NOS) guidelines [9]. Eight items were examined in total, divided into three subscales that are classified as ‘selection’, ‘comparability’ and ‘outcome/exposure’ in NOS case–control and cohort studies, and the total maximum score that can be assigned for those three subsets was 9 [9]. A study receiving a score of ≥7 was judged to be of ‘high quality’ or ‘good’. The mean value of the 17 cohort studies was 6.5 (Table S1). As a result, we had two case–control studies with a mean value of 8 that were assessed as high-quality studies, and one cross-sectional study that was considered satisfactory (Table S2 and Table S3). Similarly, the National Institutes of Health (NIH) Quality Assessment Tool for Case Series Studies) [10] was used for the assessment of case series studies; this tool examines nine elements. In the case of the three included studies, an assessment was conducted, and the studies were found to be of good quality (Table S4). Disagreements between assessments were settled following a discussion amongst the reviewing authors (SD and KP). When any additional disagreements arose, these were resolved through consultation with the third reviewing author (BH).
2.4. Outcome
Our primary outcome analysis compared the severity of the COVID-19 infection in patients with diarrhoea versus those without diarrhoea. Secondary outcomes were used to estimate the prevalence of the gastrointestinal symptoms in COVID-19 patients.
2.5. Statistical Analysis
A meta-analysis was conducted to combine the effect sizes of all included studies. software (R software version 4.1.1; R Foundation, Viena, Austria; meta, dmetar and metafor) was used to estimate the prevalence of gastrointestinal symptoms, while RevMan 5.3 (RevMan software version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration) was used for the assessment of the severity of COVID-19 in patients with diarrhoea; severely versus non-severely ill patient groups were compared. In an attempt to estimate the prevalence of the COVID-19-associated gastrointestinal symptoms, we extracted demographic data from relevant studies, sample sizes and events. Firstly, we used inverse variance to estimate the weight of the individual studies. Subsequently, the Freeman Tukey double arcsine transformation [11] was applied, thereby giving more weight to studies with a higher value than to those with a lower value. A random-effects model and an odds ratio (OR) with a 95% confidence interval (CI) were used for the assessment of the severity of COVID-19 in a patient with diarrhoea between severely and non-severely ill patient groups. To calculate the statistical heterogeneity, I2 and Cochrane’s Q test were utilised. The Forest plots represented the combined effects, while the OR was considered statistically significant when the p-value was < 0.05. Finally, funnel plots’ creation and the performance of Egger’s tests were facilitated through R software (R software version 4.1.1; R Foundation, Viena, Austria; meta, dmetar and metafor).
3. Results
A total of 1507 records were identified. After removing duplicates, 1285 records remained. After screening for titles and abstracts, 160 articles were chosen for a full-text review; of these, 72 were retrieved for further assessment. Out of the 72 articles reviewed, 16 were excluded as review articles, 15 were excluded for examining the same cohort, 13 were excluded for being systematic reviews and meta-analyses, 10 were excluded for not being full-text articles, 5 were excluded for not providing specific diarrhoea-related data, 4 were excluded for being case reports or not providing data, 2 were excluded for not providing enough data and 1 was excluded for being a case series study with no relevant data. The remaining 88 studies comprised the qualitative synthesis, while the meta-analysis included 23 studies totalling 3800 COVID-19 patients (Figure 1).
Figure 1.
PRISMA flow diagram.
Study Characteristics and Statistical Findings
Amongst the analysed 88 studies [6,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97], a total of 67,794 patients with COVID-19 infection were reported (Table 2). The countries of origin of these studies were the following: mainland China (n = 56), USA (n = 9), South Korea (n = 5), Japan (n = 2), Mexico (n = 2), Brazil (n = 1), Ethiopia (n = 1), Singapore (n = 1), Thailand (n = 1), Iraq (n = 1), France (n = 1), Italy (n = 1), Hong Kong (n = 1), Macau (n = 1), Iran (n = 2), Turkey (n = 1), Morocco (n = 1) and Malaysia (n = 1). Except for two prospective studies, most of the aforementioned studies were retrospective, with some being case–control and case series studies [33]. In COVID-19 patients, diarrhoea, abdominal pain, nausea, vomiting and loss of appetite were the most frequently reported gastrointestinal symptoms.
Table 2.
Characteristics of 88 studies: summarised overview of their demographic data, epidemiology and clinical outcomes.
| Authors | Date/Year of Publication | Study Design | Country | Sample Size N (%) | Age, Mean ±SD/Median (IQR) | Male N (%) | Female N (%) | GI Symptoms N (%) | Diarrhoea N (%) | Abdominal Pain N (%) | Nausea or Vomiting N (%) | Loss of Appetite N (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chang D, et al. [17] | March 2020 | Case series | China | 11 | 34 (34–48) | 10 | NA | 1 (7.6) | 1 (7.7) | NA | NA | NA |
| Xiong Y, et al. [74] | 22 February 2020 | Retrospective | China | 35 | 49.5 ± 14.1 | 25 (60) | NA | 10/42 (24) | 10 (24) | NA | NA | NA |
| Liu K, et al. [42] | 29 January 2020 | Retrospective | China | 137 | 57 (20–83) | 61 (44.5) | 76 (55.5) | 11 (8.0) | 11 (8.0) | NA | NA | NA |
| Guan W, et al. [27] | 28 February 2020 | Multicentre | China | 1099 | 47.0 (35.0–58.0) | 640 | 459/1096 (41.9) | ≥55 (5.0) | 42 (3.8) | NA | 55(5.0) | NA |
| Han C, et al. [29] | 31 March 2020 | Retrospective | China | 206 | 62.5 (27–92) | 91 | 115 | 117 (56.7) | 67 (32.5) | 9 (4.4) | 24 (11.7) | 102 (49.5) |
| Huang C, et al. [33] | 24 January 2020 | Prospective | China | 41 | 49.0 (41.0–58.0) | 30 (73) | 11 (27) | 1 (3.0) | 1/38 (3) | NA | NA | NA |
| Jin X, et al. [36] | 24 March 2020 | Retrospective | China | 74 | 46.14 ± 14.19 | 37 (50.0) | 74 | 74 (100) | 53 (71.62) | NA | NA | NA |
| Liu Y, et al. [44] | 9 February 2020 | Case series | China | 12 | 54.34 ± 18.011 | 8 (66.6) | 4 (33.33) | 2 (16.67) | 2 (16.67) | NA | 2 (16.67) | NA |
| Luan Y, et al. [46] | 9 July 2020 | Retrospective | China | 117 | 61.9 ± 17.9 | 62 (53.0) | 55 (47.0) | ≥8 (6.87) | 8 (6.8) | 1 (0.9) | 5(4.2) | 8 (6.8) |
| Luo S, et al. [48] | 20 March 2020 | Retrospective | China | 183 | 53.8 | 102 (56) | 81 (44) | 183 (100) | 68 (37) | 45 (25) | 37 (20) | 180 (98) |
| Ng Y, et al. [50] | 13 February 2020 | Retrospective | China | 21 | 56 (37–65) | 13 (62) | 8 (38) | 2 (9.5) | 2 (10) | NA | NA | NA |
| Pan L, et al. [52] | 14 April 2020 | Cross- sectional | China | 204 | 52.91 ± 15.98 | 107 (52.45) | 97 (47.54) | 103 (50.04) | 35 (33.98) | 2 (1.94) | 4 (3.88) | 81 (78.64) |
| Shi S, et al. [60] | 25 March 2020 | Retrospective, cohort | China | 416 | 64 (21–95) | 205 (49.27) | 211 (50.7) | 16 (3.8) | 16 (3.8) | NA | NA | NA |
| Shi H, et al. [59] | 24 February 2020 | Retrospective | China | 81 | 49.5 (11.0) | 42 (52) | 39 (38) | 4 (4.9) | 3 (4) | NA | 4 (5) | 1 (1) |
| Song F, et al. [63] | 6 February 2020 | Retrospective | China | 51 | 49 ± 16 | 25 (49) | 26 (51) | 5 (9.82) | 5 (10) | NA | 3 (6) | 9 (18) |
| An P, et al. [13] | 6 February 2020 | Retrospective | China | 9 | 35.8 (28–45) | 4 (44.44) | 5 (55.56) | 9 (100) | 1 (11.1) | 0 | 2 (22) | 6 (66.7) |
| Li K, et al. [40] | 29 February 2020 | Retrospective | China | 83 | 45.5 ± 12.3 | 44 (53.0) | 39 (47.0) | 7 (8.4) | 7 (8.4) | 7 (8.4) | NA | NA |
| Wang D, et al. [66] | 7 February 2020 | Retrospective, case series | China | 138 | 56 (42–68) | 75 (54.3) | 63 (45.7) | 55 (39.9) | 14 (10.1) | 3 (2.2) | 5 (3.6) | 55 (39.9) |
| Wang Z, et al. [69] | 16 March 2020 | Retrospective | China | 69 | 42.0 (35.0–62.0) | 32 (46) | 37 (54) | 10 (14.49) | 10 (14) | NA | 3 (4) | 7 (10) |
| Wu J, et al. [73] | 29 February 2020 | Retrospective | China | 80 | 46.1 ± 15.42 | 39 (48.75) | 41 (51.25) | 1 (1.25) | 1 (1.25) | NA | 1 (1.25) | NA |
| Xia P, et al. [75] | 31 September 2020 | Retrospective, cohort | China | 81 | 66.6 ± 11.4 | 54 (66.7) | 27 (33.3) | 26 (32.1) | 20 (24.7) | NA | 8 (9.9) | 26 (32.1) |
| Xiao F, et al. [76] | 3 March 2020 | Case series | China | 73 | 43 (0.83–7) | 41 (56.16) | 32 (43.83) | 26 (35.61) | 26 (35.61) | NA | NA | NA |
| Xu X-W, et al. [79] | 19 February 2020 | Retrospective, case series | China | 62 | 41 (32–52) | 35 (56) | 27 (44) | 3 (8) | 3 (8) | NA | NA | NA |
| Xu X, et al. [78] | 28 February 2020 | Retrospective | China | 90 | 50 (18–86) | 39 (43) | 51 (57) | 5 (6) | 5 (6) | NA | 5 (6) | NA |
| Zhang J, et al. [83] | 19 February 2020 | Retrospective | China | 140 | 57 (25–87) | 71 (50.7) | 69 (49.3) | 55/139 (39.6) | 18/139 (12.9) | 8/139 (5.8) | 24/139 (17.3) | 17/139 (12.2) |
| Zhang P, et al. [84] | 4 June 2020 | Retrospective | China | 136 | 69 (57–77) | 86 (63) | 50 (37) | 28 (21.0) | 28 (21.0) | NA | NA | NA |
| Zhao W, et al. [86] | 3 March 2020 | Retrospective | China | 101 | 44.44 (17–75) | 56 (55.4) | 45 (44.6) | 3 (3.0) | 3 (3.0) | NA | 2 (2.0) | NA |
| Zhao G, et al. [85] | 29 January 2021 | Retrospective | China | 36 | 51.24 | 13 (36.1) | 23 (63.8) | 6 (16.6) | 6 (16.6) | NA | NA | NA |
| Zheng M, et al. [88] | 19 March 2020 | Cohort | China | 68 | 47.13 (11–84) | 36 (52.94) | 32 (47.06) | 3 (4.41) | 3 (4.41) | NA | NA | NA |
| Zhou F, et al. [90] | 11 March 2020 | Retrospective, cohort | China | 191 | 56.0 (46.0–67.0) | 119 (62) | 72 (38) | 9 (4.71) | 9 (5.0) | NA | 7 (4.0) | NA |
| Zhou Z, et al. [91] | 18 March 2020 | Retrospective | China | 254 | 50 (36–65) | 115 (45.3) | 139 (54.7) | 66 (25.9) | 46 (18.1) | 3 (1.2) | 36 (14.17) | NA |
| Yang W, et al. [80] | 26 February 2020 | Retrospective, cohort | China | 149 | 45.11 ± 13.35 | 81 (54.36) | 68 (45.63) | 11 (7.38) | 11 (7.38) | NA | 2 (1.34) | NA |
| Zhang G, et al. [82] | 9 April 2020 | Retrospective, case series | China | 221 | 55.0 (39.0–66.5) | 108 (48.9) | 113 (51.1) | 80 (36.19) | 25 (11.3) | 5 (2.3) | NA | 80 (36.2) |
| Wang R, et al. [67] | 24 March 2020 | Retrospective, descriptive | China | 125 | 38.76 ± 13.799 | 71 (56.8) | 54 (43.2) | 50 (40.0) | 50 (40.0) | NA | 24 (19.2) | NA |
| Du R, et al. [23] | 7 April 2020 | Retrospective | China | 109 | 70.7 ± 10.9 | 74 (67.88) | 35 (32.1) | 29 (26.6) | 29 (26.6) | NA | NA | NA |
| Zheng T, et al. [89] | 4 June 2020 | Retrospective | China | 1320 | 50 (40–57) | 579 (43.9) | 741 (56.1) | 192 (14.54) | 107 (8.1) | 11 (0.8) | 57 (4.3) | 62 (4.7) |
| Zhao X, et al. [87] | 29 April 2020 | Retrospective | China | 91 | 46.0 | 49 (53.8) | 42 (46.2) | 19 (12.1) | 14 (15.4) | 2 (2.2) | 19 (12.1) | 11 (12.1) |
| Zhang J-J, et al. [83] | 18 February 2020 | Retrospective | China | 140 | 57 (25–87) | 71 (50.7) | 69 (49.3) | 31 (22.3) | 18 (12.9) | 8 (5.8) | 31 (22.3) | 17 (12.2) |
| Xiao Y, et al. [77] | 5 August 2020 | Descriptive | China | 90 | 61.0 (48.3–69.0) | 51 (57) | 39 (43) | 37 (41.1) | 8 (9.0) | 6 (7.0) | 37 (41.1) | 22 (24) |
| Wei X, et al. [71] | 18 July 2020 | Retrospective, single centre | China | 84 | 37 (24–74) | 28 (33) | 56 (66.6) | 26 (30.9) | 26 (30.9) | 2 (2) | 22 (26.1) | NA |
| Han J, et al. [30] | 5 August 2020 | Retrospective | China | 120 | 45.4 (15.6) | 43 (36) | 77 (64.17) | 7 (5.83) | 7 (5.83) | NA | NA | NA |
| Jiang Y, et al. [35] | 7 December 2020 | Retrospective | China | 495 | 42.24 ± 16.99 | 515 (41.5) | 723 (58.4) | 76 (15.3) | 29 (5.85) | 10 (2.02) | 35 (7.0) | 7 (1.4) |
| Lin L, et al. [41] | 2 April 2020 | Retrospective | China | 95 | 45.3 ± 18.3 | 45 (47.4) | 50 (52.6) | 58 (61) | 23 (24.2) | NA | 17 (17.9) | 17 (17.9) |
| Liu Y, et al. [43] | 18 May 2020 | Retrospective | China | 148 | 56.5 ± 15.2 | 67 (45.2) | 81 (54.7) | 42 (28.3) | 18 (12.16) | 2 (1.34) | 4 (2.7) | 27 (18.2) |
| Luo S, et al. [47] | 23 July 2020 | Retrospective cohort | China | 183 | NA | 102 (55.7) | 81 (44.2) | 183 (100) | 68 (37.1) | 65 (35.5) | 119 (65.0) | 180 (98.3) |
| Wang X, et al. [68] | 14 April 2020 | Retrospective | China | 80 | 39 (32–48.5) | 31 (38.75) | 49 (61.25) | 15 (18.75) | 15 (18.75) | NA | NA | NA |
| Chen Q, et al. [19] | 28 April 2020 | Retrospective, single centre | China | 145 | 47.5 ± 14.6 | 79 (54.5) | 66 (45.5) | 62 (42.75) | 39 (26.8) | 8 (5.5) | 30 (20.6) | 62 (42.75) |
| He S, et al. [31] | 15 October 2020 | Retrospective | China | 267 | 57 (37–68) | 116 (43) | 151 (56.5) | 20 (7) | 20 (7) | NA | NA | NA |
| Hu C, et al. [32] | 18 March 2021 | Retrospective | China | 32 | NA | 17 (53.1) | 15 (46.8) | 3 (9.4) | 3 (9.4) | NA | NA | NA |
| Tu Y, et al. [65] | 11 January 2021 | Retrospective | China | 74 | 68.0 (61.5–74.0) | 53 (71.6) | 21 (28.3) | 24 (32.4) | 24 (32.4) | 2 (2.7) | 5 (6.8) | NA |
| Wang Z H, et al. [70] | 20 July 2020 | Retrospective | China | 59 | 67.4 ± 11.3 | 38 (64.4) | 21 (35.6) | 22 (37.3) | 22 (37.3) | NA | 4 (6.8) | 11 (18.6) |
| Zheng F, et al. [81] | 10 April 2020 | Retrospective | China | 161 | 45 (33.5–57) | 80 (49.7) | 81 (50.3) | 17 (10.6) | 17 (10.6) | NA | 6 (3.7) | NA |
| Cai Q, et al. [15] | 2 April 2020 | Retrospective | China | 298 | 47.5 (33–61) | 145 (48.6) | 153 (51.3) | 9 (3.02) | 9 (3.02) | NA | NA | NA |
| Duarte-Neto A, et al. [24] | 22 May 2020 | Case Series | Brazil | 10 | 63 (33–83) | 5 (50) | 5 (50) | 2 (20) | 2 (20) | NA | NA | NA |
| Redd W, et al. [56] | 22 April 2020 | Multicentre, cohort | USA | 318 | 63.4 ± 16.6 | 174 (54.7) | 144 (45.3) | 195 (61.3) | 107 (33.7) | 46 (14.5) | 95 (29.8) | 110 (34.8) |
| Chen A, et al. [18] | 15 May 2020 | Prospective, case–control | USA | 340 | 46.89 ± 15.34 | 96 (28) | 244 (71.7) | 201 (59) | 123 (36) | 72 (21) | 135 (39.7) | 117 (34) |
| Cholankeril G, et al. [21] | 28 April 2020 | Retrospective | USA | 207 | 49 (34–65) | 104 (50.2) | 103 (49.8) | 70 (34.5) | 22 (10.8) | 14 (7.1) | 22 (10.8) | NA |
| Ramchandran P, et al. [55] | 29 June 2020 | Retrospective, cohort | USA | 31 | 57.6 ± 17.2 | 19 (61.2) | 12 (38.7) | 31 (20.6) | 15 (10) | NA | 6 (4) | NA |
| Nobel Y, et al. [51] | 12 April 2020 | Retrospective, case–control | USA | 278 | NA | 145 (52) | 133 (48) | 97 (34.8) | 56 (22.31) | NA | 63 (25.09) | NA |
| Elmunzer B, et al. [25] | 30 September 2020 | Observational, cohort | USA | 1992 | 60.1 ± 16.3 | 1128 (56.6) | 864 (43.4) | 1052 (53) | 679 (34) | 220 (11) | 539 (27) | NA |
| Ferm S, et al. [26] | 1 June 2020 | Retrospective | USA | 892 | 59 (47–72) | 534 (59.8) | 358 (40.1) | 219 (24.6) | 177 (19.8) | 70 (7.8) | 148 (16.6) | 105 (11.8) |
| Renelus B, et al. [58] | 4 September 2020 | Retrospective | USA | 734 | 66.1 ± 15.6 | 379 (51.6) | 355 (48.4) | 231 (31.5) | 149 (20.3) | 68 (9.26) | 109 (14.9) | NA |
| Kang M, et al. [37] | 13 July 2020 | Retrospective | South Korea | 118 | 61 (50–70) | 52 (44.1) | 66 (55.9) | 54 (45.8) | 54 (45.8) | NA | NA | NA |
| Banno A, et al. [14] | 9 February 2021 | Retrospective, observational | Japan | 24 | 57.5 (49–68.8) | 19 (79) | 5 (20.8) | 6 (25) | 6 (25) | NA | NA | NA |
| Remes-Troche J, et al. [57] | 21 May 2020 | Cohort | Mexico | 112 | 43.72 ± 15 | 81 (72.3) | 31 (27.7) | 23 (20.5) | 20 (17.8) | 11 (9.8) | 8 (7.1) | NA |
| Namendys-Silva S, et al. [49] | 21 October 2020 | Multicentre observational | Mexico | 164 | 57.3 ± 13.7 | 114 (69.5) | 50 (30.4) | 29 (17.6) | 29 (17.6) | NA | NA | NA |
| Sulaiman T, et al. [64] | 18 September 2020 | Retrospective | Iraq | 140 | 44.99 ± 16.81 | 100 (71.42) | 40 (28.57) | 78 (55.7) | 41 (29.28) | 42 (30) | 31 (22.14) | 40 (28.57) |
| Wolday D, et al. [72] | 14 July 2021 | Prospective, cohort | Ethiopia | 751 | 37 (28-50) | 480 (63.9) | 14 (1.9) | 76 (10.9) | 39 (5.2) | 44 (5.9) | 76 (10.9) | NA |
| Aumpan N, et al. [6] | 6 July 2020 | Retrospective | Thailand | 40 | 30.5 ± 9.2 | 18 (45) | 22 (55) | 12 (30) | 6 (15) | 2 (5) | 2 (5) | 7 (17.5) |
| Puah S, et al. [54] | 5 April 2021 | Prospective, multicentre | Singapore | 60 | 44 (41–47) | 37 (62) | 23 (38.3) | 10 (17) | 10 (17) | NA | NA | NA |
| Jang J, et al. [34] | 2 June 2020 | Retrospective | South Korea | 110 | 56.9 ± 17.0 | 48 (43.6) | 62 (56.4) | 11 (10) | 11 (10.0) | NA | 3 (2.7) | NA |
| Cheung K, et al. [20] | 3 April 2020 | Retrospective | Hong Kong | 59 | 58.5 (43.5–68) | 27 (45.7) | 32 (54.2) | 15 (25.42) | 13 (22.0) | 7 (11.9) | 1 (1.7) | NA |
| Carvalho H, et al. [22] | 4 January 2021 | Case–control | France | 1,188 | 65 (51.5–76) | 663 (55.8) | 524 (44.2) | 202 (17.0) | 202 (17.0) | NA | 137 (10.6) | NA |
| Aghemo A, et al. [12] | 10 May 2020 | Retrospective | Italy | 292 | 65 ± 14.1 | 199 (68.2) | 93 (31.8) | 69/245 (28.2) | 69/255 (27.1) | NA | 11/274 (4.0) | NA |
| Park S, et al. [53] | 10 June 2020 | Prospective | South Korea | 46 | 26 (18–57) | 21 (45.6) | 25 (54.3) | 16 (34.7) | 7 (15.2) | 5 (10.8) | 1 (2.1) | 1 (2.2) |
| Lo I, et al. [45] | 15 March 2020 | Retrospective | Macau | 10 | 54 (27–64) | 3 (30) | 7 (70) | 8 (80) | 8 (80) | 2 (20) | 5 (50) | NA |
| Kashefizadeh A, et al. [38] | 10 November 2020 | Retrospective | Iran | 53 | 58.4 ± 13.0 | 24 (45.3) | 29 (54.7) | 49 (80.8) | 32 (61.5) | 49 (80.8) | 40 (76.9) | 20 (38.5) |
| Hajifathalian K, et al. [28] | 7 May 2020 | Retrospective | USA | 1059 | 61.1 ± 18.3 | 611 (57.7) | 448 (42.3) | 827 (78) | 234 (22.1) | 72 (6.8) | 168 (15.9) | NA |
| Utku A, et al. [16] | 17 August 2020 | Cohort | Turkey | 143 | 55.63 (mean) | 77 (53.8) | 66 (46.1) | 31 (21.7) | 31 (21.7) | NA | NA | NA |
| Kim C, et al. [39] | 21 April 2021 | Retrospective | South Korea | 106 | 28 ± 9.3 | 46 (43.4) | 60 (56.6) | 7 (6.6) | 7 (6.6) | NA | NA | NA |
| Shimamura Y, et al. [61] | 16 July 2021 | Retrospective | Japan | 315 | 60 (41–74) | 179 (57) | 136 (43.1) | 45 (14.0) | 45 (14) | 45 (14) | 5 (2) | 30 (10) |
| Sim B, et al. [62] | 17 November 2020 | Observational | Malaysia | 5889 | 34.0 (24–51) | 4221 (71.7) | 1,668 (28.3) | 298 (5.1) | 298 (5.1) | NA | 108 (1.8) | NA |
| Zhao W, et al. [93] | 19 August 2022 | Retrospective | China | 208 | 53.5 ± 20.9 | 90 (44) | 118 (56) | 24 (12) | 24 (12) | 17 (8) | 15 (7) | NA |
| Lee D-S, et al. [92] | 25 May 2022 | Retrospective | South Korea | 46 | 60 (56–74) | 22 (47.8) | 24 (52.9) | 25 (54) | 25 (54) | 28 (60.8) | 11 (23.9) | NA |
| Delavari A, et al. [95] | 10 March 2022 | Retrospective | Iran | 42,964 | 51.36 ± 19.61 | 22,854 (53.2) | 20,110 (46.8) | 6356 (14.7) | 1198 (2.78) | 688 (1.60) | 1781 (4.14) | 1,638 (3.81) |
| Belabbes F-Z, et al. [97] | 9 September 2022 | Retrospective, cohort | Morocco | 154 | NA | 85 (55.2) | 69 (44.2) | 24 (15.6) | 24 (15.6) | 9 (5.8) | 8 (5.2) | 5 (3.3) |
| Sun Z, et al. [94] | 20 January 2022 | Retrospective | China | 63 | 48.0 ± 21.2 | 39 (61.9) | 24 (38.2) | 9 (14.3) | 9 (14.3) | NA | NA | NA |
| Chen D, et al. [96] | 20 October 2022 | Observational, cross-sectional | China | 93 | 58.0 ± 12.1 | 46 (49.4) | 47 (50.6) | 65 (69.5) | 27 (29.3) | NA | 26 (27.7) | 63 (67.9) |
After pooling data from these 88 studies, the prevalence of gastrointestinal symptoms in COVID-19 patients was estimated to be 27.89% (95% CI: 21.97–34.22%; I2 = 99%) (Figure S1). Following that, the prevalence of diarrhoea was estimated to be 16.93% in these 88 studies (95% CI: 14.13–19.91%; I2 = 98%) (Figure S2). The first case of COVID-19 that was associated with diarrhoea, the most commonly associated gastrointestinal symptom, was reported in China [98]. Similarly, the pooled estimate of nausea and vomiting in 60 studies was 11.93% (95% CI: 8.86–15.37%, I2 = 98%) (Figure S3). According to our analysis, the prevalence of abdominal pain was 8.89% (CI 5.25–13.30%, I2 = 98%) in 40 studies (Figure S4), and the pooled estimate of a loss of appetite was 25.13% (CI 16.18–35.26%, I2 = 99%) in 33 studies (Figure S5).
In 23 out of the examined 88 studies, [6,15,19,23,27,30,33,34,40,43,51,54,66,69,79,81,82,83,88,92,93,94,95,96,97] a COVID-19 severity assessment in patients with diarrhoea was reported. Most of the studies were conducted in China, though four papers from other countries (namely, South Korea, USA, Thailand and Singapore) have also provided such assessments. Our analysis included 3800 patients who had COVID-19. The majority of these studies compared the severely versus the non-severely ill patients, [6,15,19,27,30,34,40,43,81,82,83,88,92,93,94,96] while others compared the ICU-admitted versus the non-ICU-admitted patients [23,33,54,66]. Moreover, one study reported on patients with an SpO2 of <90% versus patients with an SpO2 of >90% [69], and another reported on patients with their symptoms’ onset being ≤10 days versus patients with their symptoms’ onset being of ≥10 days (Table 3) [79].
Table 3.
COVID-19 infection with diarrhoea: summary of severe versus non-severe cases.
| Authors | Date/Year of Publication | Study Site | Sample Size | Diarrhoea (N) | Severe Diarrhoea | Severe Total | Non-Severe Diarrhoea | Non-Severe Total | Comparison |
|---|---|---|---|---|---|---|---|---|---|
| Guan W, et al. [27] | 28 February 2020 | China | 1099 | 42 | 10 | 173 | 32 | 926 | Severe vs. non-severe |
| Li K, et al. [40] | 29 February 2020 | China | 83 | 7 | 2 | 25 | 5 | 58 | Severe vs. non-severe |
| Wang D, et al. [66] | 7 February 2020 | China | 138 | 14 | 6 | 36 | 8 | 102 | ICU vs. non-ICU |
| Wang Z, et al. [69] | 16 March 2020 | China | 69 | 10 | 2 | 14 | 8 | 55 | SpO2 ≥ 90% vs. SpO2 ≤ 90% |
| Xu X-W, et al. [79] | 19 February 2020 | China | 62 | 3 | 3 | 33 | 0 | 29 | Time since symptom onset >10 days vs. ≤10 days |
| Zheng M, et al. [88] | 19 March 2020 | China | 68 | 3 | 1 | 13 | 2 | 55 | Severe vs. mild |
| Zhang G, et al. [82] | 9 April 2020 | China | 221 | 25 | 9 | 55 | 16 | 166 | Severe vs. non-severe |
| Du R, et al. [23] | 7 April 2020 | China | 109 | 29 | 15 | 51 | 14 | 58 | ICU vs. non-ICU |
| Zhang J-J, et al. [83] | 18 February 2020 | China | 140 | 18 | 9 | 57 | 9 | 82 | Severe vs. non-severe |
| Han J, et al. [30] | 5 August 2020 | China | 120 | 7 | 5 | 30 | 2 | 90 | Severe vs. All (diarrhoea vs. no diarrhoea) |
| Liu Yu, et al. [43] | 18 May 2020 | China | 148 | 16 | 8 | 47 | 8 | 68 | Severe vs. non-severe |
| Chen Q, et al. [19] | 28 April 2020 | China | 145 | 39 | 16 | 43 | 23 | 102 | Severe vs. non-severe |
| Huang C, et al. [33] | 24 January 2020 | China | 41 | 1 | 0 | 13 | 1 | 28 | ICU vs. non-ICU |
| Zheng F, et al. [81] | March 2020 | China | 161 | 17 | 1 | 30 | 16 | 131 | Severe vs. non-severe |
| Cai Q, et al. [15] | 2 April 2020 | China | 298 | 9 | 4 | 58 | 5 | 240 | Severe vs. non-severe |
| Nobel Y, et al. [51] | 12 April 2020 | USA | 278 | 53 | 11 | 44 | 42 | 207 | Hospital admission vs. ICU admission |
| Jang J, et al. [34] | 2 June 2020 | South Korea | 110 | 11 | 1 | 23 | 10 | 87 | Severe vs. non-severe |
| Puah S, et al. [54] | 5 April 2021 | Singapore | 60 | 10 | 1 | 10 | 9 | 50 | Severe vs. mild |
| Aumpan N, et al. [6] | 6 July 2020 | Thailand | 40 | 6 | 4 | 10 | 2 | 30 | ICU vs. non-ICU |
| Zhao W, et al. [93] | 19 August 2022 | China | 208 | 24 | 4 | 20 | 20 | 188 | Severe vs. mild |
| Chen D, et al. [96] | 20 October 2022 | China | 93 | 27 | 20 | 51 | 7 | 42 | Severe vs. moderate |
| Sun Z, et al. [94] | 20 January 2022 | China | 63 | 9 | 6 | 24 | 3 | 39 | Severe vs. mild |
| Lee D-S, et al. [92] | 25 May 2022 | South Korea | 46 | 25 | 7 | 16 | 18 | 30 | Severe vs. mild |
| Total | 3800 | 405 | 145 | 876 | 260 | 2863 |
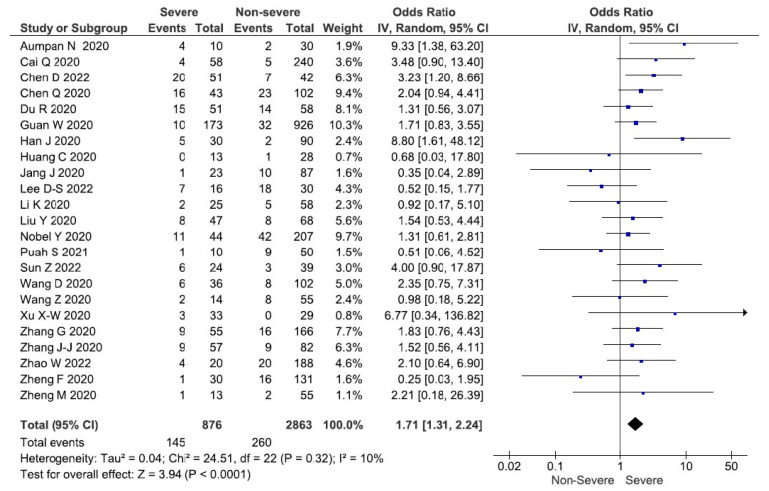
To synthesise the findings of these 23 studies, a meta-analysis using an inverse variance and a random-effects model was undertaken. The meta-analysis revealed that patients with COVID-19 and diarrhoea had an OR of 1.71 (95% CI: 1.31–2.24%; p < 0.0001) and as a result, a higher correlation with becoming severely ill. However, a minimal heterogeneity was detected amongst these studies (χ2 = 24.51, df = 22; p = 0.32, I2 = 10%) (Figure 2). A funnel plot of the included studies highlights the publication bias. The funnel plot appears to be symmetrical. Moreover, an Egger’s test was used to verify the symmetry of the funnel plot. The results showed that there is no asymmetry in the plots (t = −0.02, df = 21; p = 0.9863) for the COVID-19 patients with diarrhoea, a finding that suggests that there is no publication bias (Figure S6).
Figure 2.
Forest plot demonstrating that in COVID-19 patients who have diarrhoea is associated with more severe COVID-19 outcome when the pooled odds of patients with non-severe diarrhoea versus severe diarrhoea are compared.
4. Discussion
Patients with COVID-19 who present with diarrhoea have a higher risk of presenting with severe COVID-19. Several studies have found that the COVID-19 infection can cause gastrointestinal symptoms. Depending on the size of the sample and the number of the study sites involved, the prevalence and severity of these COVID-19-associated gastrointestinal symptoms vary. In this study, the severity assessment of COVID-19 patients with diarrhoea was compared between the severely and the non-severely ill patient groups, and the overall prevalence of COVID-19-associated gastrointestinal symptoms was computed. Out of 88 studies, 23 studies with 3800 COVID-19 patients were included for the undertaking of the meta-analysis for the delivery of the severity assessments. The chance of having a severe COVID-19 infection with diarrhoea was 1.71 (95% CI: 1.31–2.24%; p < 0.0001, I2 = 10%) times higher than those for the non-severely ill patient groups. According to the subgroup analysis, there are no significant differences in terms of value between Asian and non-Asian countries (Figure S7). As a result, we may infer that in individuals infected with COVID-19 and diarrhoea were more likely to be severely ill than those COVID-19 patients without diarrhoea. According to studies, changes in the microbiome of the gut are associated with a bidirectional shift in the interaction between the gut and a number of major organs, leading to severe disease symptoms explaining the gut–lung axis link in COVID-19 [99].
Patients with advanced age, obesity, hypertension, diabetes and dyspnoea are more likely to have severe disease [19,34,40,82]. Our meta-analysis for the severity evaluation has focused on the above risk factors. A study by Li et al., has reported that 28% of the severely ill group of COVID-19 patients had diabetes mellitus, 8% had hypertension, and 28% experienced dyspnoea [40]. This shows the relationship between comorbidities and COVID-19 severity. Moreover, studies have shown that male patients have a higher potential for developing severe COVID-19 than female patients [100,101]. After analysing the 23 included studies for severely ill COVID-19 patients with diarrhoea, our findings reveal that the pooled prevalence for male and female patients is 52.18% (95% CI: 48.67–55.67%; p < 0.01; I2 = 70%) and 47.83% (95% CI: 44.34–51.34%; p < 0.01; I2 = 70%), respectively. Hence, further analysis (that would include more data) must be performed to determine the role of sex in the severity of COVID-19.
Other parameters associated with the development of severe COVID-19 included an SpO2 of <92%, higher APACHE II and SOFA scores, bilateral lung infiltrates, decreased lymphocytes, ICU admission and prolonged hospitalisation [19,23,34,81]. In a study of 43 highly infected patients, APACHE II and SOFA scores were found to be greater in critically ill patients [19]. Further research is required to clarify the relationship between these parameters in severe COVID-19 patients with diarrhoea.
We analysed the overall prevalence of gastrointestinal symptoms in COVID-19 patients and found it to be 27%. Based on multiple studies, the most commonly reported gastrointestinal symptoms were diarrhoea, nausea, vomiting, abdominal pain and loss of appetite [52]. According to our findings, the overall prevalence of diarrhoea was 16%. However, the prevalence of diarrhoea ranged from 10% to 45% in various studies [37,102]. Diarrhoea can be the primary complaint, or it can be accompanied by fever. In a study of 103 individuals, Pan et al. reported six patients with digestive symptoms but no respiratory involvement, while 97 patients had both a digestive and respiratory involvement [52].
According to Jin et al., diarrhoea is defined as the passing of loose stool more than three times a day or as having an average of three evacuations per day [36]. Diarrhoea begins 1 to 8 days after the onset of the illness, with a median of 3.3 days; its duration ranges from 1 to 14 days, with an average of 4.1 ± 2.5 days. The maximum number of diarrhoea episodes per day is nine, while the average frequency is 3.3 ± 1.6 times per day, with 34.3% of those being watery stools [103]. There is no mucus present in the stools [104]; however, significant severe gastrointestinal symptoms are associated with blood [105]. Gastrointestinal bleeding is one of the complications of COVID-19 infection, which can possibly be confused with diarrhoea in some clinical contexts. In a study by Xiao et al., the stool features were described as yellow with no erythrocytes or leukocytes [77]. In contrast, in a study by Fang et al., leukocytes in the stool were identified in 3 out of 58 patients [103]. Owing to the disparity in results, more research is required to gain a better understanding of the stool characteristics and of their association with severe COVID-19 accompanied by diarrhoea.
Studies have also reported that symptoms are longer in diarrhoea COVID-19 patients than in those who do not have diarrhoea. As a result, it takes a longer time to eliminate SARS-CoV-2 in these patients, thereby resulting in longer hospital stays and an increased susceptibility to faecal–oral transmission [71]. According to a study conducted by Wei et al., COVID-19 patients with diarrhoea have a higher SARS-CoV-2 RNA load in their stool than those without diarrhoea [71]. A high viral load in the stool and a prolonged hospitalisation also suggest that COVID-19 patients with diarrhoea are more prone to be severely ill [71]. Several hypotheses have been proposed in an attempt to explain the occurrence of diarrhoea in COVID-19 patients [106].
According to recent review articles by Wang et al., diarrhoea could have been caused by direct viral invasion, resulting in cytotoxic damage after binding to ACE2 receptors. The attaching of SARS-CoV-2 to the ACE2 receptor can cause an ACE2 downregulation, that in turn causes a sodium-dependent glucose transport dysregulation, thereby resulting in significant gastrointestinal tract injury. An increase in proinflammatory mediators and cytokine storms may damage the digestive tract [5]. In COVID-19 patients, SARS-CoV-2 infection can cause gut dysbiosis by altering the intestinal microbiota which affect gut microbiota composition [5].
The COVID-19-associated gastrointestinal symptoms include nausea and vomiting, in addition to diarrhoea. According to our findings, 11.93% of COVID-19 patients experience nausea or vomiting. Similarly, 8% of these people experience abdominal pain, and 27% report a loss of appetite.
Several antiviral medications, such as lopinavir/ritonavir, may have contributed to this COVID-19-associated diarrhoea. According to one study, antiviral treatments for diarrhoea were ameliorated and subsequently stopped altogether [107]. In another trial, lopinavir and ritonavir did not affect the development of diarrhoea [108]. In this analysis, we looked at the use of lopinavir/ritonavir in severely infected COVID-19 individuals reported in six studies. The pooled estimate of the use of lopinavir/ritonavir in 743 severely infected COVID-19 individuals was 67.24% (95% CI: 27.38–96.33%; p < 0.01; I2 = 99%). Antiviral usage is fairly prevalent in patients with severe COVID-19; hence, more research is needed to establish if antivirals are linked to the development of diarrhoea in severe COVID-19 patients.
Recently, favipiravir has been linked to diarrhoea. According to the WHO database, 7% of 93 people who took favipiravir had diarrhoea [109]. In one of the herein analysed studies, favipiravir was used for the treatment of severe COVID-19 infections [15]. Remdesivir, in contrast, was not included in any of the studies. However, remdesivir has been linked to diarrhoea in 9% of the patients receiving it [110]. Additional research is needed to determine whether these antivirals are associated with severe COVID-19-associated diarrhoea. Other drugs, such as molnupiravir (a novel antiviral medication), have been linked to the development of headaches and diarrhoea [111]. In both the placebo and molnupiravir-receiving groups, multiple molnupiravir doses were associated with a 7.1% chance of developing diarrhoea [112]. The use of monoclonal antibodies in the form of a bamlanivimab monotherapy for the treatment of COVID-19 has been shown to cause nausea and diarrhoea in 1% of those receiving 700 mg, in 1.9% of those receiving 2800 mg and in 5.9% of those receiving 7000 mg; in contrast, combination therapy of bamlanivimab with etesevimab has been reported to cause diarrhoea in 1% of the patients receiving it [113]. Additional research on these novel antiviral treatments and monoclonal antibodies will be needed in the future to gain a better understanding of their role in generating diarrhoea in severely ill COVID-19 patients.
There were 23 studies that were included in our analysis. There was no information on vaccine administration in any of these trials. According to our findings, one study was linked to the B1.1.7 variant epidemic [54]. Aside from fever, cough and sore throat, B1.1.7 variants are known to trigger gastrointestinal problems in a limited number of people. European studies have reported diarrhoea and abdominal pain with the B1.1.7 variant [114]. In one particular study, the B1.6.1.7 (delta strain) was shown to cause abdominal pain, nausea, vomiting and diarrhoea [115]. In a study by Wang et al., 17 out of 25 critically ill COVID-19 patients experienced diarrhoea when infected with the delta strain [107]. However, of the 38 individuals who presented with severe symptoms, only 8 were found to be infected with the delta strain. None of the patients in the Wang et.al study had diarrhoea, but one in eight (twelve) had nausea and vomiting [116]. Because of the small sample size, it is difficult to establish whether there is a link between diarrhoea and specific COVID-19 variants. Based on past research, we may conclude that COVID-19 mutations are linked to gastrointestinal problems. The undertaking of further study is required to determine the degree of disease severity with these variants (including the omicron variant) in COVID-19; however, no relevant reports on the omicron variant have been published so far.
4.1. The Implications of the Study
COVID-19 individuals who experienced diarrhoea had an increased likelihood of being severely ill. This might be due to the existence of a gut–lung axis. Similarly, COVID-19 patients presented with gastrointestinal symptoms (27%) and diarrhoea (16%). Approximately 35% of those who have COVID-19 had diarrhoea as their first symptom, with no respiratory involvement. These findings aid physicians in raising awareness of the gastrointestinal involvement during the COVID-19 outbreak. The severity of COVID-19 is also related to the individual’s age and other comorbidities. The use of both new and old antiviral drugs can produce diarrhoea in most of patients, but further study is required to determine the relationship between antiviral usage and the severity of COVID-19-associated diarrhoea. The development of diarrhoea has also been linked to the use of monoclonal antibodies, while a variety of genetic mutations has also been associated with the development of diarrhoea.
4.2. Limitation of the Study
Our study has certain limitations; our research includes only papers written in English. There are several excluded articles that lack data on the disease severity and diarrhoea. The majority of the data included in the meta-analysis were from Asian countries. There was also a lack of data on antiviral drugs that may have influenced the disease severity, as well as a lack of data regarding the association of the undertaken vaccination and certain variants with diarrhoea and disease severity.
5. Conclusions
Our meta-analysis provides further evidence to support the hypothesis that the risk of developing severe COVID-19 in patients with diarrhoea is higher than in COVID-19 patients who did not have diarrhoea based on the study data during the COVID-19 pandemic. COVID-19 patients with gastrointestinal symptoms account for 27% of the cases, with diarrhoea being a symptom in 16% of COVID-19 patients. Physicians should also raise awareness that diarrhoea in COVID-19 might be associated with a more severe clinical course, and some COVID-19 patients might be presented with gastrointestinal symptoms. During the COVID-19 pandemic, clinicians should consider COVID-19 as a possible diagnosis for cases with gastrointestinal symptoms. Finally, clinical observation and early medical treatment should be prioritised when a patient is diagnosed with COVID-19-associated diarrhoea.
Acknowledgments
We would like to express our heartfelt appreciation to the Department of Clinical Tropical Medicine of Mahidol University (Thailand) for assisting and encouraging us throughout this work.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/tropicalmed8020084/s1, Figure S1: Pooled prevalence of overall gastrointestinal symptoms among 88 included studies; Figure S2: A pooled estimate of the prevalence of diarrhoea symptoms in COVID-19 infected patients; Figure S3: A pooled estimate of the prevalence of nausea and vomiting symptoms in COVID-19 infected patients; Figure S4: A pooled estimate of the prevalence of abdominal pain symptoms in COVID-19 infected patients; Figure S5: A pooled estimate of the prevalence of loss of appetite symptoms in COVID-19 infected patients; Figure S6: A funnel plot for publication bias for the severity of diarrhoea in COVID-19 infected patients; Figure S7: Forest plot demonstrating sub-groups analysis of COVID-19-infected patients’ diarrhoea in Asian versus non-Asian groups: title; Table S1: Newcastle–Ottawa scale (NOS) summary assessment of the risk of bias in 16 cohort studies; Table S2: Newcastle–Ottawa scale (NOS) summary assessment of the risk of bias in 2 case-control studies; Table S3: Newcastle-Ottawa scale (NOS) summary assessment of the risk of bias for a cross-sectional study; Table S4: National Institutes of Health (NIH) study quality assessment tool for risk of bias for case-series studies.
Author Contributions
S.D., K.P. and P.C. participated in the study design and execution; S.D., K.P., P.C., W.P.-n., V.L., C.S., B.H. and S.C. gathered, analysed and interpreted the data; S.D. and K.P. made contributions to the manuscript writing; S.D., K.P., P.C., W.P.-n., V.L., B.H. and C.S. critically analysed the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study has been exempted by the International Review Board of the Faculty of Tropical Medicine, Mahidol University, Thailand (TMEC 21-024).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The study was funded by the Faculty of Tropical Medicine of Mahidol University. The funders had no role in the study’s design, data collection and interpretation, publication or manuscript writing.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Elkhatib W.F., Abdelkareem S.S., Khalaf W.S. Narrative review on century of respiratory pandemics from Spanish flu to COVID-19 and impact of nanotechnology on COVID-19 diagnosis and immune system boosting. Virol. J. 2022;19:167. doi: 10.1186/s12985-022-01902-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant M.C., Geoghegan L., Arbyn M. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2; COVID-19): A systematic review and meta-analysis of 148 studies from 9 countries. PLoS ONE. 2020;15:e0234765. doi: 10.1371/journal.pone.0234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klopfenstein T., Kadiane-Oussou N.D.J., Royer P.-Y. Diarrhea: An underestimated symptom in Coronavirus disease 2019. Clin. Res. Hepatol. Gastroenterol. 2020;44:282–283. doi: 10.1016/j.clinre.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozkurt Z., Çınar Tanrıverdi E. COVID-19: Gastrointestinal manifestations, liver injury and recommendations. World J. Clin. Cases. 2022;10:1140–1163. doi: 10.12998/wjcc.v10.i4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M.-K., Yue H.-Y., Cai J. COVID-19 and the digestive system: A comprehensive review. World J. Clin. Cases. 2021;9:3796–3813. doi: 10.12998/wjcc.v9.i16.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aumpan N., Nunanan P., Vilaichone R.K. Gastrointestinal manifestation as clinical predictor of severe COVID-19: A retrospective experience and literature review of COVID -19 in Association of Southeast Asian Nations (ASEAN) JGH Open. 2020;4:1096–1101. doi: 10.1002/jgh3.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghimire S., Sharma S., Patel A. Diarrhea Is Associated with Increased Severity of Disease in COVID-19: Systemic Review and Metaanalysis. SN Compr. Clin. Med. 2021;3:28–35. doi: 10.1007/s42399-020-00662-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berlin D.A., Gulick R.M., Martinez F.J. Severe COVID-19. N. Engl. J. Med. 2020;383:2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 9.The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. [(accessed on 23 September 2021)]. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 10.Study Quality Assessment Tools|NHLBI, NIH. [(accessed on 25 September 2021)]; Available online: https://www.ncbi.nlm.nih.gov/pubmed/
- 11.Lin L., Xu C. Arcsine-based transformations for meta-analysis of proportions: Pros, cons, and alternatives. Health Sci. Rep. 2020;3:e178. doi: 10.1002/hsr2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aghemo A., Piovani D., Parigi T.L. COVID-19 Digestive System Involvement and Clinical Outcomes in a Large Academic Hospital in Milan, Italy. Clin. Gastroenterol. Hepatol. 2020;18:2366–2368. doi: 10.1016/j.cgh.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An P., Chen H., Jiang X. Clinical Features of 2019 Novel Coronavirus Pneumonia Presented Gastrointestinal Symptoms But Without Fever Onset. SSRN Electron. J. 2020 doi: 10.2139/ssrn.3532530. [DOI] [Google Scholar]
- 14.Banno A., Hifumi T., Okamoto H. Clinical characteristics and outcomes of critically ill COVID-19 patients in Tokyo: A single-center observational study from the first wave. BMC Infect. Dis. 2021;21:163. doi: 10.1186/s12879-021-05840-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai Q., Huang D., Ou P. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742–1752. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 16.Utku A.C., Budak G., Karabay O. Main symptoms in patients presenting in the COVID-19 period. Scott. Med. J. 2020;65:127–132. doi: 10.1177/0036933020949253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang D., Lin M., Wei L. Epidemiologic and Clinical Characteristics of Novel Coronavirus Infections Involving 13 Patients Outside Wuhan, China. JAMA. 2020;323:1092. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen A., Agarwal A., Ravindran N. Are Gastrointestinal Symptoms Specific for Coronavirus 2019 Infection? A Prospective Case-Control Study From the United States. Gastroenterology. 2020;159:1161–1163. doi: 10.1053/j.gastro.2020.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Q., Zheng Z., Zhang C. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection. 2020;48:543–551. doi: 10.1007/s15010-020-01432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung K.S., Hung I.F.N., Chan P.P.Y. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cholankeril G., Podboy A., Aivaliotis V.I. Association of Digestive Symptoms and Hospitalization in Patients With SARS-CoV-2 Infection. Am. J. Gastroenterol. 2020;115:1129–1132. doi: 10.14309/ajg.0000000000000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Carvalho H., Richard M.C., Chouihed T. Electrolyte imbalance in COVID-19 patients admitted to the Emergency Department: A case–control study. Intern. Emerg. Med. 2021;16:1945–1950. doi: 10.1007/s11739-021-02632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du R.H., Liu L.M., Yin W. Hospitalization and Critical Care of 109 Decedents with COVID-19 Pneumonia in Wuhan, China. Ann. Am. Thorac. Soc. 2020;17:839–846. doi: 10.1513/AnnalsATS.202003-225OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duarte-Neto A.N., Monteiro R.A.A., Silva L.F.F. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology. 2020;77:186–197. doi: 10.1111/his.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elmunzer B.J., Spitzer R.L., Foster L.D. Digestive Manifestations in Patients Hospitalized With Coronavirus Disease 2019. Clin. Gastroenterol. Hepatol. 2020;19:1355–1365. doi: 10.1016/j.cgh.2020.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferm S., Fisher C., Pakala T. Analysis of Gastrointestinal and Hepatic Manifestations of SARS-CoV-2 Infection in 892 Patients in Queens, NY. Clin. Gastroenterol. Hepatol. 2020;18:2378–2379. doi: 10.1016/j.cgh.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan W.-J., Ni Z.-Y., Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hajifathalian K., Krisko T., Mehta A. Gastrointestinal and Hepatic Manifestations of 2019 Novel Coronavirus Disease in a Large Cohort of Infected Patients From New York: Clinical Implications. Gastroenterology. 2020;159:1137–1140. doi: 10.1053/j.gastro.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han C., Duan C., Zhang S. Digestive Symptoms in COVID-19 Patients With Mild Disease Severity: Clinical Presentation, Stool Viral RNA Testing, and Outcomes. Am. J. Gastroenterol. 2020;115:916–923. doi: 10.14309/ajg.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han J., Gong H., Fu L. Clinical and CT imaging features of SARS-CoV-2 patients presented with diarrhea. J. Infect. 2020;81:e33–e35. doi: 10.1016/j.jinf.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He S., Zhou K., Hu M. Clinical characteristics of “re-positive” discharged COVID-19 pneumonia patients in Wuhan, China. Sci. Rep. 2020;10:17365. doi: 10.1038/s41598-020-74284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu C., Li J., Xing X. The effect of age on the clinical and immune characteristics of critically ill patients with COVID-19: A preliminary report. PLoS ONE. 2021;16:e0248675. doi: 10.1371/journal.pone.0248675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jang J.G., Hur J., Choi E.Y. Prognostic Factors for Severe Coronavirus Disease 2019 in Daegu, Korea. J. Korean Med. Sci. 2020;35:e209. doi: 10.3346/jkms.2020.35.e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang Y., Han C., Bai T. Gastroenterologists Reveal More Digestive Symptoms in COVID-19 Patients than Nongastroenterologists in Fever Clinic. Gastroenterol. Res. Pract. 2020;2020:8853922. doi: 10.1155/2020/8853922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin X., Lian J.-S., Hu J.-H. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69:1002–1009. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang M.K., Kim K.O., Kim M.C. Clinical characteristics of coronavirus disease 2019 patients with diarrhea in Daegu. Korean J. Intern. Med. 2020;35:1261–1269. doi: 10.3904/kjim.2020.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kashefizadeh A., Ohadi L., Golmohammadi M. Clinical features and short-term outcomes of COVID-19 in Tehran, Iran: An analysis of mortality and hospital stay. Acta Biomed. 2020;91:e2020147. doi: 10.23750/abm.v91i4.10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim C., Kim W., Jeon J.H. COVID-19 infection with asymptomatic or mild disease severity in young patients: Clinical course and association between prevalence of pneumonia and viral load. PLoS ONE. 2021;16:e0250358. doi: 10.1371/journal.pone.0250358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li K., Wu J., Wu F. The Clinical and Chest CT Features Associated With Severe and Critical COVID-19 Pneumonia. Invest. Radiol. 2020;55:327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin L., Jiang X., Zhang Z. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 42.Liu K., Fang Y.Y., Deng Y. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin. Med. J. 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Y., Xiang L., Deng K. Focusing on Gastrointestinal Symptoms in COVID-19 Is Far From Enough. Gastroenterology. 2021;160:1429–1430. doi: 10.1053/j.gastro.2020.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y., Yang Y., Zhang C. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. Chin. Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lo I.L., Lio C.F., Cheong H.H. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int. J. Biol. Sci. 2020;16:1698–1707. doi: 10.7150/ijbs.45357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luan Y.-Y., Liu Y., Liu X.-Y. Coronavirus disease 2019 (COVID-19) associated coagulopathy and its impact on outcomes in Shenzhen, China: A retrospective cohort study. Thromb. Res. 2020;195:62–68. doi: 10.1016/j.thromres.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo S., Deng Z., Zhang X. Clinical characteristics and outcomes of 2019 novel coronavirus disease patients presenting with initial gastrointestinal symptoms in Wuhan, China: A retrospective cohort study. J. Gastroenterol. Hepatol. 2021;36:694–699. doi: 10.1111/jgh.15199. [DOI] [PubMed] [Google Scholar]
- 48.Luo S., Zhang X., Xu H. Don’t Overlook Digestive Symptoms in Patients With 2019 Novel Coronavirus Disease (COVID-19) Clin. Gastroenterol. Hepatol. 2020;18:1636–1637. doi: 10.1016/j.cgh.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ñamendys-Silva S.A., Alvarado-Ávila P.E., Domínguez-Cherit G. Outcomes of patients with COVID-19 in the intensive care unit in Mexico: A multicenter observational study. Heart Lung. 2021;50:28–32. doi: 10.1016/j.hrtlng.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ng M.-Y., Lee E.Y.P., Yang J. Imaging Profile of the COVID-19 Infection: Radiologic Findings and Literature Review. Radiol. Cardiothorac. Imaging. 2020;2:e200034. doi: 10.1148/ryct.2020200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nobel Y.R., Phipps M., Zucker J. Gastrointestinal Symptoms and Coronavirus Disease 2019: A Case-Control Study From the United States. Gastroenterology. 2020;159:373–375. doi: 10.1053/j.gastro.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan L., Mu M., Yang P. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am. J. Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park S.-K., Lee C.-W., Park D.-I. Detection of SARS-CoV-2 in Fecal Samples From Patients With Asymptomatic and Mild COVID-19 in Korea. Clin. Gastroenterol. Hepatol. 2020;19:1387–1394. doi: 10.1016/j.cgh.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Puah S.H., Young B.E., Chia P.Y. Clinical features and predictors of severity in COVID-19 patients with critical illness in Singapore. Sci. Rep. 2021;11:7477. doi: 10.1038/s41598-021-81377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramachandran P., Onukogu I., Ghanta S. Gastrointestinal Symptoms and Outcomes in Hospitalized Coronavirus Disease 2019 Patients. Dig. Dis. 2020;38:373–379. doi: 10.1159/000509774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Redd W.D., Zhou J.C., Hathorn K.E. Prevalence and Characteristics of Gastrointestinal Symptoms in Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection in the United States: A Multicenter Cohort Study. Gastroenterology. 2020;159:765–767. doi: 10.1053/j.gastro.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Remes-Troche J.M., Ramos-De-La-Medina A., Manríquez-Reyes M. Initial Gastrointestinal Manifestations in Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection in 112 Patients From Veracruz in Southeastern Mexico. Gastroenterology. 2020;159:1179–1181. doi: 10.1053/j.gastro.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Renelus B.D., Khoury N., Chandrasekaran K. Hospitalized coronavirus disease-2019 (COVID-19) patients with gastrointestinal symptoms have improved survival to discharge. Dig. Liver Dis. 2020;52:1403–1406. doi: 10.1016/j.dld.2020.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect. Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi S., Qin M., Shen B. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shimamura Y., Masuda K., Anbo Y. A single-center comparative analysis of outpatients with and without COVID-19 in Sapporo, Japan. J. Gen. Fam. Med. 2021;23:61–64. doi: 10.1002/jgf2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sim B.L.H., Chidambaram S.K., Wong X.C. Clinical characteristics and risk factors for severe COVID-19 infections in Malaysia: A nationwide observational study. Lancet Reg. Health—West. Pac. 2020;4:100055. doi: 10.1016/j.lanwpc.2020.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song F., Shi N., Shan F. Emerging 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology. 2020;295:210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sulaiman T., Algharawi A.A., Idrees M. The prevalence of gastrointestinal symptoms among patients with COVID -19 and the effect on the severity of the disease. JGH Open. 2020;4:1162–1166. doi: 10.1002/jgh3.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tu Y., Yang P., Zhou Y. Risk factors for mortality of critically ill patients with COVID-19 receiving invasive ventilation. Int. J. Med. Sci. 2021;18:1198–1206. doi: 10.7150/ijms.50039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang D., Hu B., Hu C. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang R., Pan M., Zhang X. Epidemiological and clinical features of 125 Hospitalized Patients with COVID-19 in Fuyang, Anhui, China. Int. J. Infect. Dis. 2020;95:421–428. doi: 10.1016/j.ijid.2020.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang X., Liu W., Zhao J. Clinical characteristics of 80 hospitalized frontline medical workers infected with COVID-19 in Wuhan, China. J. Hosp. Infect. 2020;105:399–403. doi: 10.1016/j.jhin.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Z., Yang B., Li Q. Clinical Features of 69 Cases With Coronavirus Disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020;71:769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Z.-H., Shu C., Ran X. Critically Ill Patients with Coronavirus Disease 2019 in a Designated ICU: Clinical Features and Predictors for Mortality. Risk Manag. Healthc. Policy. 2020;13:833–845. doi: 10.2147/RMHP.S263095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei X.-S., Wang X., Niu Y.-R. Diarrhea Is Associated With Prolonged Symptoms and Viral Carriage in Corona Virus Disease 2019. Clin. Gastroenterol. Hepatol. 2020;18:1753–1759. doi: 10.1016/j.cgh.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wolday D., Gebrecherkos T., Arefaine Z.G. Effect of co-infection with intestinal parasites on COVID-19 severity: A prospective observational cohort study. EClinicalMedicine. 2021;39:101054. doi: 10.1016/j.eclinm.2021.101054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu J., Liu J., Zhao X. Clinical Characteristics of Imported Cases of Coronavirus Disease 2019 (COVID-19) in Jiangsu Province: A Multicenter Descriptive Study. Clin. Infect. Dis. 2020;71:706–712. doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu J., Wu X., Zeng W., Chest C.T. Findings in Patients With Coronavirus Disease 2019 and Its Relationship With Clinical Features. Invest. Radiol. 2020;55:257–261. doi: 10.1097/RLI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xia P., Wen Y., Duan Y. Clinicopathological Features and Outcomes of Acute Kidney Injury in Critically Ill COVID-19 with Prolonged Disease Course: A Retrospective Cohort. J. Am. Soc. Nephrol. 2020;31:2205–2221. doi: 10.1681/ASN.2020040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xiao F., Tang M., Zheng X. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao Y., Huang S., Yan L. Clinical characteristics of diarrhea in 90 cases with COVID-19: A descriptive study. Int. Emerg. Nurs. 2020;52:100912. doi: 10.1016/j.ienj.2020.100912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xu X., Yu C., Qu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur. J. Nucl. Med. Mol. Imaging. 2020;47:1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu X.-W., Wu X.-X., Jiang X.-G. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-CoV -2) outside of Wuhan, China: Retrospective case series. BMJ. 2020;368:m792. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang W., Cao Q., Qin L. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):A multi-center study in Wenzhou city, Zhejiang, China. J. Infect. 2020;80:388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zg F.Z., Tang W. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur. Rev. Med. Pharmacol. Sci. 2020;24:3404–3410. doi: 10.26355/eurrev_202003_20711. [DOI] [PubMed] [Google Scholar]
- 82.Zhang G., Hu C., Luo L. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J. Clin. Virol. 2020;127:104364. doi: 10.1016/j.jcv.2020.104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang J.-J., Dong X., Cao Y.-Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 84.Zhang P., He Z., Yu G. The modified NUTRIC score can be used for nutritional risk assessment as well as prognosis prediction in critically ill COVID-19 patients. Clin. Nutr. 2021;40:534–541. doi: 10.1016/j.clnu.2020.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao G., Xu Y., Li J. Sex differences in immune responses to SARS-CoV-2 in patients with COVID-19. Biosci. Rep. 2021;41:BSR20202074. doi: 10.1042/BSR20202074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao W., Zhong Z., Xie X. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. Am. J. Roentgenol. 2020;214:1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 87.Zhao X.-Y., Xu X.-X., Yin H.-S. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: A retrospective study. BMC Infect. Dis. 2020;20:311. doi: 10.1186/s12879-020-05010-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng M., Gao Y., Wang G. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zheng T., Yang C., Wang H.Y. Clinical characteristics and outcomes of COVID-19 patients with gastrointestinal symptoms admitted to Jianghan Fangcang Shelter Hospital in Wuhan, China. J. Med. Virol. 2020;92:2735–2741. doi: 10.1002/jmv.26146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou Z., Zhao N., Shu Y. Effect of Gastrointestinal Symptoms in Patients With COVID-19. Gastroenterology. 2020;158:2294–2297. doi: 10.1053/j.gastro.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee D.S., Kim J.W., Lee K.L. Significance of digestive symptoms after COVID-19 vaccination: A retrospective single-center study. Am. J. Emerg. Med. 2022;58:154–158. doi: 10.1016/j.ajem.2022.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao W., Li Y., Xie R. Real-World Evidence for COVID-19 Delta Variant’s Effects on the Digestive System and Protection of Inactivated Vaccines from a Medical Center in Yangzhou, China: A Retrospective Observational Study. Int. J. Clin. Pract. 2022;2022:7405448. doi: 10.1155/2022/7405448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun Z., Song Z.-G., Liu C. Gut microbiome alterations and gut barrier dysfunction are associated with host immune homeostasis in COVID-19 patients. BMC Med. 2022;20:24. doi: 10.1186/s12916-021-02212-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Delavari A., Asgari S., Alimohamadi Y. Gastrointestinal symptoms are associated with a lower risk of hospitalization and mortality and outcomes in COVID-19. BMC Gastroenterol. 2022;22:119. doi: 10.1186/s12876-022-02190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen D., Ning M., Feng Y. The early stage of COVID-19 pandemic: Gastrointestinal manifestations and liver injury in COVID-19 patients in Wuhan, China. Front. Med. 2022;9:997000. doi: 10.3389/fmed.2022.997000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Belabbes F.Z., Maizi M., Belghyti N. Prevalence and Severity of Gastrointestinal Symptoms in COVID-19 Patients in Casablanca: A Retrospective Cohort Study. Cureus. 2022;14:e27815. doi: 10.7759/cureus.27815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang X., Zhao J., Yan Q. A case of COVID-19 patient with the diarrhea as initial symptom and literature review. Clin. Res. Hepatol. Gastroenterol. 2020;44:e109–e112. doi: 10.1016/j.clinre.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Malik J.A., Ahmed S., Yaseen Z. Association of SARS-CoV-2 and Polypharmacy with Gut–Lung Axis: From Pathogenesis to Treatment. ACS Omega. 2022;7:33651–33665. doi: 10.1021/acsomega.2c02524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Booth A., Reed A.B., Ponzo S. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS ONE. 2021;16:e0247461. doi: 10.1371/journal.pone.0247461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hu J., Wang Y. The Clinical Characteristics and Risk Factors of Severe COVID-19. Gerontology. 2021;67:255–266. doi: 10.1159/000513400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cha M.H., Regueiro M., Sandhu D.S. Gastrointestinal and hepatic manifestations of COVID-19: A comprehensive review. World J. Gastroenterol. 2020;26:2323–2331. doi: 10.3748/wjg.v26.i19.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fang D., Ma J., Guan J., Wang M., Song Y., Tian D., Li P. Manifestations of digestive system of hospitalized patients with coronavirus disease 2019 in Wuhan, China: A single-center descriptive study. Zhonghua Xiaohua Zazhi. Chin. J. Dig. 2020;12:151–156. [Google Scholar]
- 104.D’Amico F., Baumgart D.C., Danese S. Diarrhea During COVID-19 Infection: Pathogenesis, Epidemiology, Prevention, and Management. Clin. Gastroenterol. Hepatol. 2020;18:1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Carvalho A., Alqusairi R., Adams A. SARS-CoV-2 Gastrointestinal Infection Causing Hemorrhagic Colitis: Implications for Detection and Transmission of COVID-19 Disease. Am. J. Gastroenterol. 2020;115:942–946. doi: 10.14309/ajg.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang H., Kang Z., Gong H. Digestive system is a potential route of COVID-19: An analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut. 2020;69:1010–1018. doi: 10.1136/gutjnl-2020-320953. [DOI] [Google Scholar]
- 107.Song Y., Liu P., Shi X.L. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut. 2020;69:1143–1144. doi: 10.1136/gutjnl-2020-320891. [DOI] [PubMed] [Google Scholar]
- 108.Wan Y., Li J., Shen L. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet Gastroenterol. Hepatol. 2020;5:534–535. doi: 10.1016/S2468-1253(20)30118-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kaur R.J., Charan J., Dutta S. Favipiravir Use in COVID-19: Analysis of Suspected Adverse Drug Events Reported in the WHO Database. Infect. Drug Resist. 2020;13:4427–4438. doi: 10.2147/IDR.S287934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grein J., Ohmagari N., Shin D. Compassionate Use of Remdesivir for Patients with Severe COVID-19. N. Engl. J. Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rusu A., Arbănaşi E.-M., Lungu I.-A. Perspectives on Antiviral Drugs Development in the Treatment of COVID-19. Acta Biol. Marisiensis. 2021;4:44–59. doi: 10.2478/abmj-2021-0005. [DOI] [Google Scholar]
- 112.Painter W.P., Holman W., Bush J.A. Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity Against SARS-CoV-2. Antimicrob. Agents Chemother. 2021;65:e02428-20. doi: 10.1128/AAC.02428-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gottlieb R.L., Nirula A., Chen P. Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19. JAMA. 2021;325:632–644. doi: 10.1001/jama.2021.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Graham M.S., Sudre C.H., May A. Changes in symptomatology, reinfection, and transmissibility associated with the SARS-CoV-2 variant B.1.1.7: An ecological study. Lancet Public Health. 2021;6:e335–e345. doi: 10.1016/S2468-2667(21)00055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Williams H., Hutchinson D., Stone H. Watching Brief: The evolution and impact of COVID-19 variants B. 1.1. 7, B. 1.351, P. 1 and B. 1.617. Glob. Biosecurity. 2021;3 doi: 10.31646/gbio.112. [DOI] [Google Scholar]
- 116.Wang Y., Chen R., Hu F. Transmission, viral kinetics and clinical characteristics of the emergent SARS-CoV-2 Delta VOC in Guangzhou, China. EClinicalMedicine. 2021;40:101129. doi: 10.1016/j.eclinm.2021.101129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.