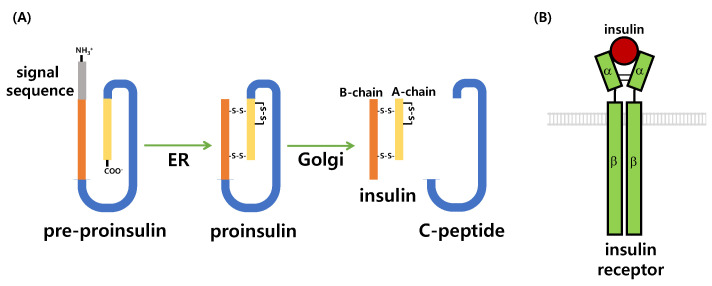
Figure 6.
Insulin synthesis and insulin receptor. (A) Insulin biosynthesis begins as a precursor, preproinsulin, within the pancreatic β cell cytosol. Preproinsulin is comprised N-terminal signal sequence (gray), B-chain (orange), C-peptide (blue), and A-chain (yellow). Preproinsulin translocates into the endoplasmic reticulum and by cleavage of the signal sequence, forms proinsulin. The proinsulin folds by forming 3 disulfide bonds then trafficking through Golgi complex into secretory granules. Prohormone convertase and carboxypeptidase E processes proinsulin to C-peptide and mature insulin composed with A- and B-chain. ER: endoplasmic reticulum. (B) Insulin receptor is a dimer of identical units that span the cell membrane. Each of the 2 subunits is made of α-chain and β-chain, connected by a single disulfide bond. The α-chain is located extracellular and binds with insulin. The β-chain spans the cell membrane and has a tyrosine kinase domain which is activated when insulin binds to the α-chain.