Abstract
The Vaccinium L. (Ericaceae) genus consists of a globally widespread and diverse genus of around 4250 species, of which the most valuable is the Vaccinioidae subfamily. The current review focuses on the distribution, history, bioactive compounds, and health-related effects of three species: cranberry, blueberry, and huckleberry. Several studies highlight that the consumption of Vaccinium spp. presents numerous beneficial health-related outcomes, including antioxidant, antimicrobial, anti-inflammatory, and protective effects against diabetes, obesity, cancer, neurodegenerative diseases and cardiovascular disorders. These plants’ prevalence and commercial value have enhanced in the past several years; thus, the generated by-products have also increased. Consequently, the identified phenolic compounds found in the discarded leaves of these plants are also presented, and their impact on health and economic value is discussed. The main bioactive compounds identified in this genus belong to anthocyanins (cyanidin, malvidin, and delphinidin), flavonoids (quercetin, isoquercetin, and astragalin), phenolic acids (gallic, p-Coumaric, cinnamic, syringic, ferulic, and caffeic acids), and iridoids.
Keywords: Vaccinium uliginosum L., Ericaceae, herbal medicine, bioactivity, in vitro and in vivo analyses, phytochemical analysis, drug discovery, traditional medicine
1. Introduction
Fruit production has been growing steadily in the last few decades based on the data reported by the United Nations Food and Agriculture Organization [1]. On the other hand, as the population is also increasing, and following recent trends regarding consumer preferences for organic, good-quality food products and healthy everyday living, the generated fruits are insufficient to provide the best quality of food globally [2,3]. Additionally, as fruit production increases, the generation of large quantities of by-products is inevitable [4,5,6]. Integrating these by-products into functional foods presents a promising alternative to reduce the generated waste and increase several foodstuffs’ nutritional quality [7,8,9,10,11].
Belonging to the Ericaceae (Rhododendron) genus, the Vaccinium species comprise a morphologically diverse genus of 4250 species (33 types), divided into 9 subfamilies and 125 genera prevalent across Europe, South East and Central Africa, Asia, and North and Central America [12,13,14]. The wild species of this genus that are specific to Europe are V. myrtillus L. (bilberry), V. vitis-idaea L. (lingonberry), V. oxycoccus L. (cranberry), and V. uliginosum L. (bog bilberry) [15]. The most cultivated species with the highest economic value belong to the subfamily of Vaccinioidae, and are particularly represented by cranberry, blueberry, huckleberry, and bilberry [16]. Due to its various health-related benefits, its consumption is in constant growth, being efficient in relation to diabetes, obesity, cardiovascular and neurodegenerative diseases, atherosclerosis, rheumatoid arthritis, and cancer [17,18]. Based on several studies, the leading health benefits are accredited to their antioxidant, antimicrobial, and detoxifying effects on the organism [19,20,21]. Additionally, it is considered that these plants, particularly those with high polyphenol quantity such as anthocyanins, are able to hinder and initiate apoptosis within cancer cells [22].
The importance of polyphenols from plants has been acknowledged in the last few years, mainly due to them being an essential element for the human microbiota. As they are characterized as prebiotics, “a substrate that is selectively utilized by host microorganisms conferring a health benefit,” they bestow protective properties on the cardiovascular, immune, gastrointestinal, and central nervous systems [23,24,25]. The principal aspects of their interest are related to the current pandemic, which has highlighted the importance of a strong immune system [26] and the efficiency of food packaging, such as active packaging enriched with bioactive compounds [27,28,29,30].
Vaccinium is a common and widespread genus of shrubs or dwarf shrubs in the heath family (Ericaceae). Humans eat the fruits of many species, and some are of commercial importance, including cranberry, blueberry, bilberry (whortleberry), lingonberry (cowberry), and huckleberry. These species, including their flowers, leaves, and fruits, are widely applied in traditional medicine [31]. The content of phenolic compounds in these plants is considerably affected by the level of development, the localization of the plant, and by genetic causes. An important element is the amount of sunlight, which greatly influences the quantity of phenolic compounds [32]. Similar to other types of plants, the fruits are the parts most often consumed, while the other parts, such as leaves and stems, are discarded [33,34,35].
The current review aims to describe the current knowledge regarding the species cranberry, blueberry, and huckleberry belonging to the Vaccinium genus; their fruits, leaves, and stems; and their phytochemistry and biological properties, with specific attention paid to their health-related effects and economic value.
2. Blueberry/Bilberry (V. myrtillus/V. sect. cyanococcus)
2.1. History, Nomenclature, and Production
Bilberry (V. myrtillus) is a perennial low-growing shrub which can grow up to 35–60 centimetres in height. It grows in acidic soils [36], organic forest soils, mountainous mineral heaths, and old peat bogs in central and northern parts of Europe [37]. The shrubs can also be found in parts of Asia and North America, where it is located under the name of blueberry (V. corymbosum L.). Bilberry is usually known as European blueberry or whortleberry [36]. V. myrtillus is a diploid shrub [15] which blooms from April through June. It produces spheroidal blue or black intensely coloured fruits that can grow up to 5–9 millimetres in diameter, with many seeds, that mature from July to September. The pulp has the shade of the peel, and the taste is sweet with astringent notes. An uncommon albino form is characterized by greenish-white fruits, where this appearance is caused by the overthrow of genes that code the anthocyanin synthesis [38]. Bilberry is generally known as a “super food”, thanks to its abundance in healthful compounds and its employment in human diet from ancient times. The first written shreds of evidence of decoction preparations from dried bilberry fruits to apply in folk medicine date from the 18th century [36].
The reputation of bilberry can be attributed to its balanced sweet and sour flavour and rich nutritional value. Bilberries collected from wild areas are consumed and commercially available mostly as berries (fresh, dried, and frozen) and in the form of canned industrial products, including jams, juices, and concentrates. The fruits are utilized, in combination with other products, to produce syrups, pies, tarts, and beverages. Leaves are generally used in order to produce decoctions [36,39]. The expanding demand for a berry-rich diet has led to an expansion in the consumption and management of two Vaccinium genus berries. Those breeds are the V. myrtillus, or wild berries, and V. corymbosum, or cultivated berries [40]. Over the last five years, the production of blueberry has mainly been focused in North America [41], accounting for over 80% of world production [42], and with a production that reached over 1 million (M) tons in 2021 [40,43]. The U.S. registered a 106% increase in blueberry production from 2007 to 2016 [42]. According to FAOSTAT, in 2020, a total cultivated blueberry area of 126.144 ha was recorded globally; a significant difference compared to 1980, when the cultivated area was 24.460 ha. Previously discussed data indicate an increase of approximately 303% in the last 20 years.
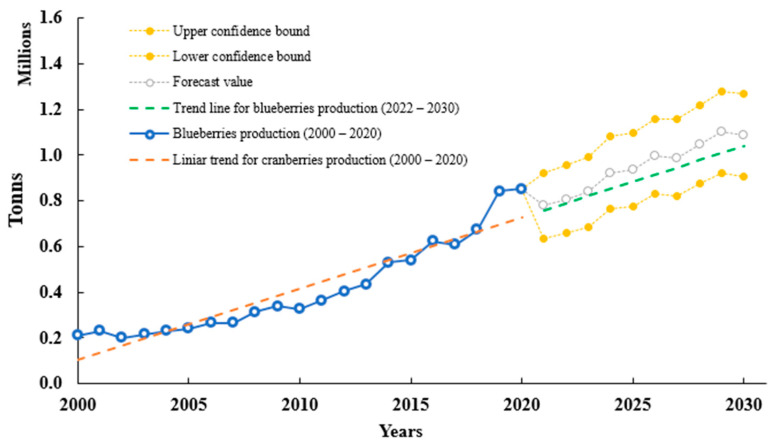
Blueberry production and consumption are predicted to go through the same interest and constant growth, enlarging by 22%; more accurately, over 1 M tons by 2030 [1]. In addition, considering the last 20 years of blueberry production and using Excel 2022 and FORECAST function, we predicted blueberry production until 2030 based on existing values that follow a seasonal trend. A confidence interval was estimated using interval values from 2000–2020. The interval was defined by its lower and upper bounds from 2020 until 2030. Seasonality was considered to be one decade. The confidence interval is expressed as a percentage (95%). The percentage reflects the confidence level. According to data analysis from FAO data, the blueberry forecast may reach a value of 1.08 ± 0.26 M tons by 2030. Figure 1 shows a constant forecast and trendline that will increase in the following years.
Figure 1.
Evolution of world blueberry production over time. According to FAO, global blueberry production (tons) from 2000 until 2030 and TREND production until 2030. The trendline was calculated considering blueberry production every year from 2000 to 2030. The TREND function returns values along with a linear trend. It fits a straight line (using the method of least squares) to the arrays known_ys and known_xs. TREND returns the y-values along that line for the array of new_xs that you specify.
2.2. Blueberry Constituents
Bilberries incorporate high amounts of sugars (68.5–205.3 g/kg), organic acids (5.7–14.2 g/kg), and a mixture of phenolic compounds with multiple biological effects. Anti-carcinogenic, antimicrobial, and anti-inflammatory activities were indicated by in vitro studies performed on bilberries [39]. In terms of proximate composition, fresh bilberry fruits contain 84% water, 9.7% carbohydrates, 0.6% proteins, 0.4% fats, and 3–3.5% fibres, and their energetic value is estimated to be about 192 kJ/100 g [44,45].
Phenolic compounds represent the primary group of phytochemicals found in berries. They acquire one or more aromatic rings with hydroxyl groups, and their construction may range from a simple phenolic molecule to a complex high-molecular-mass polymer, as can be seen in Table 1, adapted from [32,38,46]. Many factors can determine the content of phenolic compounds in the aerial parts of berry plants; these can be genotype, growing conditions, ripeness, and storage conditions. Researchers demonstrated that the fruits of berry plants grown in a cold climate with a short vegetation season are characterized by a superior content of phenolic compounds rather than the same varieties that are grown in a moderate climate. Phenolic compounds can be used to determine authenticity, since genotype profoundly impacts the qualitative and quantitative composition of these compounds in berries [47].
Table 1.
Phenolic compounds identified in aerial parts of Vaccinium plants.
| Class | Phenolic Compounds | Chemical Structures (Main Compound) | |
|---|---|---|---|
| Leaves | Stems | ||
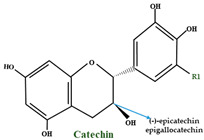
| Catechins | (+)-catechin—R1 = H (–)-epicatechin—R1 = H+ gallocatechin—R1 = OH epigallocatechin—R1 = OH |
|
|
| Cinchonains | cinchonains I cinchonains II |
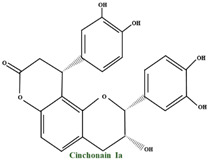
|
|
| Phenolic acids | 3,4–dihydroxybenzoic p–coumaroyl quinic acid isomers p–coumaroyl malonic acid p–coumaroyl derivatives p–coumaroyl glucose coumaroyl iridoid |
|
|
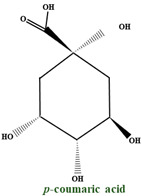
| p–coumaric acid | - |
|
|
| feruloyl quinic acid isomer | - | ||
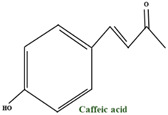
| caffeoyl quinic acid isomers | - | ||
| caffeic acid ethyl ester | - | ||
| caffeic acid hexoside | - | ||
| Proantho- cyanidins |
B–type dimer B–type trimer B–type tetramer B–type pentamer A–type dimer A–type trimer procyanidin A2 procyanidin B1 procyanidin B2 |
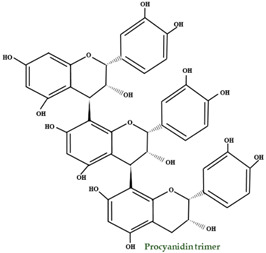
|
|
| Flavonols | quercetin–3–O–(4”–HMG)–α–rhamnoside quercetin–3–O–galactoside quercetin–3–O–glucoside quercetin–3–O–rutinoside quercetin–3–O-α–rhamnoside quercetin–3–O–arabinoside quercetin–3–O–glucuronide |
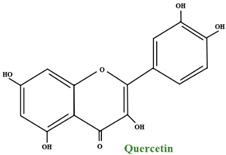
|
|
| kaempferol | - |
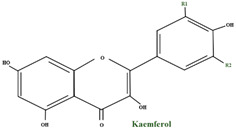
|
|
| kaempferol–3–glucuronide | - | ||
| kaempferol–hexoside | - | ||
| kaempferol–O–pentoside | - | ||
| kaempferol–(HMG)–rhamnoside | - | ||
| Lignans | - | Lyoniside (9–O–β–D–xylopyranosyl(+)lyoniresinol) |
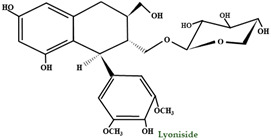
|
2.2.1. Anthocyanins
Anthocyanins are the primary components isolated and identified in berries (reaching up to 0.1–0.25% of the fresh fruits) and leaves, along with diverse, active constituents, including: resveratrol, flavonols (quercetin, catechins), phenolic acids, ellagitannins, and iridoids [36]. The fruits are defined by the existence of a complex mixture of over 20 different anthocyanins, each comprised of sugar(s) and an aglycone. Glucose and rhamnose are the most frequent sugar moieties hooked to the aglycone, but galactose, xylose, rutinose, arabinose and other sugars can also be found in that linkage. The anthocyanidins present a significant skeleton of the 2–phenyl–1–benzopyrylium cation. These form an entire group of phenolic substances characterized as plant secondary metabolites that determine the intense colouration of distinctive plant organs, mainly flowers, fruits, and the products obtained from them. Anthocyanins represent phenolic secondary metabolites associated with the large family of phenylchroman derivatives (flavonoid family). They are glycosides of anthocyanidins, of which the most significant in the plants are: petunidin, malvidin, peonidin, pelargonidin, delphinidin, and cyanidin, in a distribution of 7, 7, 12, 12, 12, and 50%, respectively [47,48].
Bilberries (V. myrtillus L.) represent the richest natural source of anthocyanins and possess the highest content of them (300–700 mg/100 g of fresh fruit), but the content may vary depending on the degree of ripeness, edaphoclimatic conditions, and cultivars. These components belonging to the flavonoid family confer bilberries their blue/black colour and high antioxidant properties [15]. V. myrtillus contains primarily delphinidin and cyanidin in a 1:1 proportion, followed by petunidin, peonidin, and malvidin, with a discrepancy in the substitution pattern of ring B. At least three distinctive sugars (β–D–glucose, β–D–galactose, and α–L–arabinose) are glycosidically associated with the C3 position. Inconsequential compounds are associated with the groups of flavan-3-ols and their respective glycosides, iridoids, phenolic acids, terpenes, organic acids, stilbenes, and pectins. Additionally, 1% of tannins (generally proanthocyanidins (PACs) and fewer amounts of ellagitannins) are found [36,38,48].
V. myrtillus possesses the most intensely coloured berries thanks to the fact that both peel and pulp contain a massive amount of anthocyanidins (up to 2% of the fresh mass in peels). Anthocyanins constitute about 90% of the total phenolic compounds in bilberry fruits. These fruits contain distinctive cyanidin- and delphinidin–3-O–sambubiosides, wherein the saccharide moiety is glucose (2 → 1) xylose. The isolation of 3-O–methyl anthocyanidins in large quantities from bilberry fruits is also feasible [38].
Anthocyanins from bilberry extracts are so essential that they have been regulated at quantities of 32 and 40% of anthocyanidins (aglycon of anthocyanins), as reported by the 8th edition of European Pharmacopoeia (2014) [49]. Furthermore, the glycosylated form of cyaniding and delphinidin accounts for 60% of the total amount of anthocyanins. Two studies delineated the berry anthocyanin metabolism. They stated that fifteen minutes following the ingestion of bilberry anthocyanins, there was an increase in the plasma level of the particles from 2.5 mg/L (400 mg/kg per os) to 50 mg/L (100 mg/kg for ethanol extract), and afterwards, a significant decline was seen. The primary anthocyanins in the plasma were malvidin–3–glucoside and malvidin–3–galactoside. Furthermore, anthocyanidins are characterized by a specific organotropism, particularly prevalent in the liver, kidney, testes, and lungs, but absent in the brain, heart, fat, muscle, and eyes [2]. Regarding its health effects, anthocyanins are described as a bioactive key capable of many reported benefits [50].
2.2.2. Flavonols
From the flavonol point of view, quercetin is the principal flavonol of bilberry fruits, representing more than 50% of the total flavonoid content. The second most plentiful flavonol found in fruits is myricetin. Other flavonols found in low levels are: laricitrin, syringetin, and isorhamnetin. Kaempferol, another component of the flavonols family, is found only in trace amounts in fruits but in bountiful quantities in leaves. In V. myrtillus, flavonols develop primarily in glycosylated form, and studies showed diverse hexosides, pentosides, and glucuronides. The main glycosides found in bilberry fruits are rhamnosides and glucuronides. Furthermore, phlorizin, a glucoside of a dihydrochalcone phloretin, was found in these fruits, but the aglycone itself was not identified [38].
2.2.3. Tannins, Procyanidins, and Organic Acids
All the plants of the Vaccinium genus represent one of the prosperous sources of tannins of both hydrolyzable and condensed types [51]. A B–type dimer of catechin was found in fruits, but in both leaves and fruits, epicatechin and catechin are found in abundance. Epigallocatechin and gallocatechin can be found mainly in leaves. Both fruits and leaves of bilberry (V. myrtillus) are abundant in procyanidin in diversified dimers and trimers. The universal B–type procyanidins are characterized by a single connection between structural units of catechins, while the rare A–type procyanidins by a double bond. The A–type linkage is dominant over the B–type in bilberries, which is abnormal in other plant species. V. myrtillus leaves and fruits represent a rich source of organic acids and their derivatives. Past studies showed that most of the total phenolic compounds are found in the leaves, and in the fruits, they are overshadowed by anthocyanins. The simple organic acids present in bilberry fruits are shikimic, citric, malic, and quinic. The composition of phenolic and hydroxycinnamic acids is dominated by p-coumaric, caffeic, and ferulic acids, followed by gallic, ellagic and syringic acids. In the fruits, vanillic acid, salicylic acid, and dihydroxybenzoic acid can also be found, but in a smaller amount. The organic acids mentioned above can be found either free, etherified, or esterified. The etherification process usually occurs with various saccharide fractions, and they can develop esters with each other or with different phenolic compounds. The most distinguished of these by-products are chlorogenic and neochlorogenic acids, which regularly make up the bulk of the total phenolic acids of bilberry fruits and leaves [38].
2.3. Antioxidant Effect
Attention to phenolic compounds has grown recently, mainly because they are exceptional antioxidants [52]. The consumption of antioxidants has proven very efficient in avoiding cancer, cardiovascular diseases, osteoporosis, diabetes, obesity, and skin ageing. The fields where the antioxidant properties of plant phenolic compounds are relevant are food (inhibition of lipid oxidation), physiology (protection against oxidative stress), and cosmetology. The antioxidant characteristics of polyphenols have been linked to their capacity to act as reducing agents. They reverse the UV filter and can connect with metal ions and proteins. Mainly, phenolic compounds produce antioxidant activity by precisely decreasing reactive oxygen species (ROS), restraining the enzymes that are participating in oxidative stress, associating metal ions engaged for the creation of ROS, and strengthening endogenous antioxidant defence systems. Dróżdż, Šėžienė, and Pyrzynska concluded that bilberry fruits displayed high antioxidant activity as suitable electron donors and their extracts were capable of lowering copper (II)-neocuproine chelate, as well as extinguishing 2,2–diphenyl–1–picrylhydrazyl radicals (DPPH) [32,44].
Principally, phenolic compounds employ their antioxidant activity by directly scavenging ROS, restraining the enzymes participating in oxidative stress, reconstructing of other antioxidants (α–tocopherol), chelating metal ions involved in ROS production, and incentivising the endogenous antioxidant defence system. Partial reduction of oxygen generates ROS, which develops radical oxygen species, such as anion superoxide, hydroxyl radical, nitric oxide, oxyl, and peroxyl radicals (peroxidation process of lipids). The most notorious approaches for the determination of the antioxidant activity of phenolic extracts are the Folin–Ciocalteu method, DPPH (2,2–diphenyl–1–picrylhydrazyl) radical scavenging method, oxygen radical absorbance capacity (ORAC), ferric-ion-reducing antioxidant power (FRAP), and Trolox, which involves equivalent antioxidant capacity (TEAC) and cupric ion reducing antioxidant capacity (CUPRAC). The Folin–Ciocalteu method uses gallic acid to access the total phenolic concept (TPC) from the bilberry extract. Additionally, it evaluates the ability of a bilberry sample to diminish the transition of the metal ions, similar to the complex between sodium phosphomolybdate and phosphotungstate. The DPPH test is based on the competence of lowering the number of molecules to transfer an electron or a hydrogen atom to the nitrogen-centred DPPH radical. The peroxyl radical is performed with a fluorescent probe for the oxygen radical absorbance capacity (ORAC) procedure to create a nonfluorescent product. That product is quantitated by fluorescence. The FRAP, TEAC, and CUPRAC procedures represent electron transfer-based tests, which modify colour when bis (neocuprine) Cu2+Cl2, Fe3+ (2,4,6–tripyridyl–s–triazine) 2Cl3, and 2,2–azinobis (3–ethylbenzothiazoline–6-sulfonic acid) radical cation (ABTS+) probes are diminished. The antioxidant capacity of the leaves and stems of bilberry was highlighted as a result of their capability to lower the DPPH radical and transition metal ions in the Folin–Ciocalteu assay, and a defensive effect against polyunsaturated dietary lipids in the first steps of gastric digestion. Bilberry leaves present an antioxidant potential (79.30 and 59.58 mM TE/100 g DM in ABTS and FRAP tests) more substantial than that of fruits (35.34 and 26.81 mM TE/100 g DM in ABTS and FRAP assays). On the fruits and leaves of V. myrtillus, water, ethyl, and ethanol extraction were performed to test their antioxidant activity. The results showed that the water extract from leaves possesses high antioxidant activity, which is in interrelationship with the large concentration of total phenols [32].
2.4. Antimicrobial Activity
It is acknowledged that most phenolic substances possess antibacterial properties. The most relevant are flavonoids, phenolic acids, tannins, and lignans [53]. Regarding bilberry, it has been proven that the antimicrobial activity is determined by the flavonoid fraction, especially anthocyanins. The bilberry extract (BE), along with other purified polyphenolic extracts of berries, presents antimicrobial effects against human pathogenic bacteria, including Salmonella and Staphylococcus aureus [54]. Bilberry phenolic extracts from fruits and leaves possess antimicrobial effects, principally in avoiding urinary tract infections (UTI) [32,55].
Moreover, BE demonstrated competence in inhibiting Helicobacter pylori expansion and some distinct Gram-positive bacteria: Bacillus, Clostridium, and Staphylococcus strains. Conversely, there is evidence that pure phenolic compounds can restrict and inhibit only Gram-negative bacteria such as Salmonella species and Escherichia coli. Furthermore, the hydroxylation activity of the phenolic compounds is linked to the Gram-negative bacteria enumerated before [45]. Hence, it can be expected that phytocomplexes or whole fruits perform more efficiently as antimicrobials, with all respect for the purified compounds [36].
Toivanen et al. established that juices obtained from V. myrtillus exhibit potential contra pneumococcal infections activated by Neisseria meningitides with a 63% increase in inhibition at a concentration of 10 mg/mL [56]. Huttunen et al. focused on the inhibitory action of wild berry juice fragments constructed mainly of sugars and small amounts of small-size phenolics on Streptococcus pneumoniae linked to human bronchial cells. They concluded that the highest concentration in their antimicrobial assay (over 85 mg/g) was exceptionally effective, and the development of Streptococcus pneumoniae was inhibited by the extract of V. myrtillus [44,57]. Another study on the antimicrobial effects of the lipid extracts of two berries, bilberry and lingonberry, by Klavins et al. showed that the fractionated lipids of both berries possess antimicrobial activity. Bilberry and lingonberry lipids were extracted from their seeds using CHCl3. The texture and presentation of the extracted lingonberry lipids were a semisolid yellow-coloured paste, while the lipids extracted for bilberry presented a wet texture and a dark green colour [58]. The obtained extraction return was 2.84 g of lipids/100g for bilberry and 2.22 g/100g for lingonberry. Larger seeds than lingonberry characterize bilberries, which represent the fruit’s energy storage, and fatty acid contents are higher; up to over 60% of the lipids. Because of the size of the seeds, extraction yield in the case of bilberries was higher than lingonberries. Lingonberry incorporates elevated amounts of alkanes and alkenes (17%), isoprenoids (17%), and phenolic acids (31%). Meanwhile, bilberry contains higher amounts of di–ketones (3%), which are not found in lingonberries [58].
The antimicrobial activity of total berry lipid extracts and extract fractions was tested against six human or opportunistic pathogens using the agar-well diffusion method. Microorganisms used in this assay were: S. aureus MSCL 334, Streptococcus pyogenes MSCL 620, S. epidermitis MSCL 333, E. coli MSCL 332, Pseudomonas aeruginosa MSCL 331, and Proteus mirabilis MSCL 590. The antibiotic used for the positive control was gentamicin (15 mg/mL). All the samples subjected to tests were at a concentration of 15 mg/mL. The results showed that the obtained fractions from bilberry and lingonberry presented antimicrobial activity against S. aureus, S. pyogenes, S. epidermidis, and E. coli, but showed no effect against P. mirabilis and P. aeruginosa. The inhibition zones reached about 20 mm for bilberry chloroform fraction with S. aureus. The antibiotic used for the positive control, gentamicin, exhibited powerful antimicrobial effects on all tested bacterial strains. The solvent used for the negative control was DMSO (Dimethylsulfoxide) [58].
BE and its fractions, principally rich in β–sitosterol, containing 280 mg/g and 70 mg/g, exhibited high inhibition potential against S. aureus strains. Therefore, chloroform and ethyl acetate fractions had 20 and 15 mm inhibition zones. The chloroform and ethyl fractions were also characterized by an inhibitory effect against other Gram-positive bacteria, including S. pyogenes and S. epidermidis. All tested bilberry fractions showed an inhibitory effect against S. pyogenes, except the most non-polar hexane fraction. The tested berry lipid fractions had an inhibitory effect against only one Gram-negative bacteria, E. coli. Inhibition zones of 11 mm were presented by the bilberry ethyl acetate/methanol and methanol fraction [58].
2.5. Health Benefits
V. myrtillus fruits have been consumed since ancient times and have become popular nowadays, representing an essential part of the everyday diet and used in diverse popular medicines [59]. These berries are well known as plentiful natural sources of polyphenols and more bioactive compounds with health effects. The consumption of bilberry fruits presents a broad spectrum of health benefits, such as antioxidant, anti-proliferative, anti-inflammatory, anti-obesity, cardioprotective, hypoglycemic, ocular, and antibacterial effects, as presented in Table 2 [44,60,61,62].
Table 2.
Traditional uses of V. myrtillus.
| Traditional Uses | Used Parts | Administration | References |
|---|---|---|---|
| Fevers and coughs | Fruits | 50–200 mg/kg | [63] |
| Respiratory inflammations | Leaves/fruits | [58,64] | |
| Antidiabetic | Leaves | 10 mg | [65] |
| Anti-inflammatory | Leaves Fruits |
4 mg/mL 100 ng/mL |
[66] [67] |
| α-glucosidase activity | Fruits | 20 μg GAE/mL ME 55 μg GAE/mL AE |
[61] |
| Neuroprotective effects | Fruits | 100 mg/kg | [68] |
| Digestive Urinary tract disorders |
Fruits | 250 mg GAE/L | [22] |
| Eye inflammation | Fruits | 50 mg/mL | [69] |
| Ulcerative colitis | Fruits | 95 g DW | [70] |
| Hypertension Weight gain reduction |
Fruits | 3% (w/w) 10% (w/w) |
[71] |
| Sun Protection Factor (SPF) | Fruits | 2.84 g/100 gDW | [58] |
| Diabetic retinopathy | Leaves/fruits | 100 mg/kg | [72] |
| Antiseptic, astringent, tonic | Fruits | 28.3 μg CYA-3-GLU equivalents/mL CM |
[73] |
| Angiotensin-Converting Enzyme Activity | Fruits | 0.1 mg/mL | [74] |
GAE—gallic acid equivalents; DW—dry weight; CM—culture medium; ME—methanol extract; AE—aqueous extract.
2.5.1. Anti-Inflammatory Effect
The anti-inflammatory activities of bilberry fruits and their extracts are based on their high anthocyanin content. Mirtoselect is a commercial BE standardized to a concentration of anthocyanins of 40%. This extract showed a complex anti-inflammatory response in lipopolysaccharide-activated macrophages. Its activity consists of attenuating pro-inflammatory cytokines (including TNF–α, IL–1β, IL–6, and COX–2) and enfeeblement of multiple lipopolysaccharide-induced chemokines and IL receptors almost to control levels [75].
The feedback of the human THP–1 monocytic cell after the treatment with anthocyanin-rich bilberry extract to inflammatory stimulation was more diverse. BE attenuated the IFN–γ–induced signal protein activation, cytokine secretion, and pro-inflammatory gene expression, since it increased TNF–α–induced reactions [67]. This effect offers a distinct role of anthocyanins in inflammatory modulation that can diversify from target to target. In vitro results are presented in various clinical studies based on inflammatory diseases; for example, after ingesting 500 g of bilberries daily, patients diagnosed with gingivitis presented reduced inflammatory cytokine levels and minor bleeding on probing, representing a routine dental clinical parameter of inflammation [76]. In another study performed on patients suffering from increased cardiovascular risk, after ingesting 330 mL of bilberry juice daily for four weeks, some of the participants’ inflammatory blood markers substantially decreased in comparison with the placebo group (C–reactive protein, IL–6, IL–15, and monokine induced by INFγ), although others remained unaffected. Surprisingly, an expansion of TNF–α was also observed [77].
Subjects with metabolic syndrome were tested after eating 400 g of fresh bilberry for eight weeks. Reductions of several inflammatory parameters (C-reactive protein, IL–6, IL–12, and LPS) were registered and typically showed lower inflammation scores [76]. In metabolic syndrome subjects, complementary anti-inflammatory evidence was accomplished with a supplement consisting of purified bilberry and blackcurrant anthocyanins Accordingly, it is a fair assumption that these components are the predominant active principle behind the sympathetic effects of V. myrtillus on cardiometabolic risk factors [77].
Inflammatory bowel disease, a functional gastrointestinal disorder [78], often lacks proper treatment, having several adverse effects. The necessity to find a suitable and effective treatment is reasonable. Evidence shows that a dysregulated immune response referring to intestinal microbiota causes the inflammation. Experiments in vitro showed that bilberry fruit extract and the isolated anthocyanins could constrict the inflammatory response of human colon epithelial cell cultures aroused by IFN–γ/IL–1β/TNF–α [77]. An open, non-controlled, and non-blinded trial performed on patients with moderate ulcerative colitis revealed that over 90% of the subjects presented positive feedback to a blend made of dried bilberry powder and concentrated bilberry juice, with a number over 60% of the patients that achieved remission [38,70].
Several diseases are influenced in their development and progression by inflammation, both acute and chronic. Schink et al. [62], in a selection of 99 ethanolic plant extracts, established that V. myrtillus presented intense anti-inflammatory activity linked with high cell viability in THP–1, HeLa–TLR4, and HEKTLR2/HEK–TLR4 cell lines. Anthocyanin-rich extracts from V. myrtillus displayed anti-inflammatory effects contra liver inflammation in mice, conducting to the suppression of LPS-induced inducible nitric oxide synthase (iNOS), TNF–α, IL–1β, and IL–6 transcripts, and iNOS, TNF–α, and NF–κB protein levels [63]. Moreover, bilberry also showed its ability to decrease serum C-reactive protein (CRP), IL–6, IL–12, and LPS levels, and lowered genes linked with the TLR pathway in individuals who presented with metabolic syndrome [44,62,76].
2.5.2. Anti-Cancer and Anti-Proliferative Activity
Several studies performed on bilberry extracts to determine their antioxidant activity helped create treatment programs against different types of cancer. Portions containing flavonoids, catechins, and organic acids extracted from bilberry fruits were potent in inhibiting the development of cervix epitheloid carcinoma, breast carcinoma, and colon adenocarcinoma cell lines (with IC50 extending between 125 to 300 mg/mL) [79]. The growth of three colon cancer cell lines (Caco–2, HT–29, and HCT–116) was restrained by the whole bilberry extract [22]. Additionally, the progression of prostate cancer in cell lines was obstructed with whole bilberry extract and the standardized mixture of its principal phenolic compounds [73].
The powder obtained from the whole bilberry suppressed the proliferation, viability, invasion, and migration of oral squamous carcinoma cell lines in the zebrafish model [80]. The fruit extract also prohibited early preneoplastic liver cell lesions in rats [38,81].
2.5.3. Antidiabetic Effect
Studies performed on human individuals showed that fasting serum hippuric acid is enhanced after ingesting anthocyanin-rich bilberries. This can assist in the beneficial effect of bilberry consumption through its ability to improve cell function and glycaemic control in diabetic patients [60]. Angiogenesis represents the physiological progression through which the circulatory system is improved by developing new blood vessels from pre-existing ones made in the earlier stage of vasculogenesis. The improvement of the vasculature is protracted by angiogenesis with processes of sprouting and splitting [82]. Studies demonstrated that irregular angiogenesis is the cause of multiple ocular diseases, such as neovascular glaucoma, diabetic retinopathy, and age-related macular degeneration [83]. In accordance, bilberries exhibit an inhibitory effect on angiogenesis and can involve indirect antidiabetic properties [83,84].
Bilberries possess the ability to take an angiotensin-converting enzyme role in human angothelial cells and demonstrated their effectiveness on cardiovascular disease together with their antidiabetic properties [74]. Studies revealed that bilberry’s inhibitory property on angiogenesis results from the effects of their principal components, including malvidin, delphinidin, and cyaniding [84].
V. myrtillus leaf has a notable role in reducing hyperglycaemia in diabetic animals [85]. They can delay or even inhibit the initial stage of early diabetic retinopathy. Furthermore, bilberries enhanced diabetes-induced endothelial permeability in addition to their linked loss of tight junction integrity and vascular endothelial growth factor (VEGF) upregulation [72]. α–glucosidase represents one of the most important enzymes linked with diabetic disease, and studies reported that bilberries could inhibit α–glucosidase enzyme [65]. Bilberries can be considered a treatment strategy because they significantly reduce oxidative stress in rats [86]. A study showed that the consumption of bilberry could improve insulin secretion and decrease blood glucose [31].
Studies performed on animal models to show bilberry’s antidiabetic activity demonstrated that blood glucose was meaningfully lowered after a treatment session with a phenolic-rich extract and an anthocyanin-enriched part from bilberry extract in mice [87]. One study by Asgary et al. [88] demonstrated the antihyperlipidemic and anti-hyperglycaemic effects of bilberry fruits in alloxan-induced diabetic rats. Lowering the branched-chain amino acids and upgraded lipid formation was stated as the principal antidiabetic mechanism of bilberries [89], alongside the capability of its leaves to treat dyslipidaemiae linked with impaired TG-rich lipoprotein clearance in rats [86]. V. myrtillus fruits lowered markers of diabetic retinopathy, such as (VEGF) expression and degradation of zonula occludens–1 and claudin–5 in diabetic rats. In conclusion, bilberry extract has the ability to prevent or delay the beginning of early diabetic retinopathy [64,72].
2.5.4. Anti-Obesity Effect
The anti-obesity techniques of bilberries may comprise a decline in lipid absorption, a diminish in proliferation and differentiation of preadipocytes, a cutback in lipogenesis, an escalation in lipolysis, and restriction of pro-inflammatory adipokine secretion [90,91]. The ability of V. myrtillus to decrease lipid aggregation with an adjuvant down-regulation of peroxisome proliferator-activated receptor gamma (PPARγ) was presented by Kowalska et al. [90]. Together with PPARγ, other elements were found in mouse embryo 3T3–L1 adipocytes, including: enhancer-binding protein alpha (C/EBPα) and sterol regulatory element binding transcription factor 1 (SREBP1c). Another ability of bilberries found by the researchers was the potential to restrain the expression of adipocyte fatty acid-linking protein (aP2) and resistin [44,92].
2.5.5. Cardioprotective Activity
Cardiovascular diseases (CVD) represent one of the most dominant causes of death and are a fundamental focus of research and treatment [44,93]. Atherosclerosis is a chronic inflammatory disorder linked with oxidative processes. It represents the principal cause of CVD, along with myocardial infarction (MI), stroke, heart failure, and claudication [94]. An investigation performed on an apolipoprotein E–deficient (apo E–/–) mice model of atherosclerosis showed that a dietary supplement composed of bilberry anthocyanin-rich extract, including over 50% of pure anthocyanins ingested for two weeks, was able to lower plasmatic total cholesterol (about 20%) and hepatic triglyceride levels (about 30% in the liver). However, the plasma antioxidant capacity remained unaltered [95]. Moreover, another study on the same subject showed that these bilberry extracts act on the inflection of gene expression implicated in angiogenesis in the aortas of apo E–/– mice [96]. The potential favourable consequence of V. myrtillus has also been studied on the evolution of obesity in mice subject to a high-fat diet (HFD) [71]. The animals were fed with 5% or 10% (w/w) of whole bilberries in HFD for about three months. They presented a reduced glucose level and lower blood pressure related to mice fed only with HFD. Furthermore, adding bilberries to HFD showed a reduced level of various inflammation parameters, but the insulin status in mice was not affected by adding bilberries to their diet.
Studies performed on humans showed that bilberry leaves possess a cardioprotective activity [97]. Erlund et al. [98] conducted human research to examine the effects of consuming a mixture composed of 100 g of bilberries and nectar containing 50 g of crushed lingonberries for two months, and the mix was consumed daily. The study was performed on well-established risk factors of CVD: platelet function, HDL cholesterol, and blood pressure. Furthermore, the subjects ingested strawberry puree or blackcurrant and cold-pressed chokeberry and raspberry juice on alternating days. No significant changes were observed in plasma biomarkers of platelet activation, fibrinolysis, or coagulation. Meaningful changes were registered in the systolic blood pressure, which was lowered, and the serum HDL cholesterol concentrations were raised. A recent study presented critical changes in lipid metabolites caused by an intake of bilberry in combination with fish and wholegrain in subjects suffering from coronary diseases [32,99].
3. Cranberry (V. sect. Oxycoccus)
3.1. History, Nomenclature and Production
Cranberry is a diploid fruit [100], a woody perennial that produces vertical stems [101], belonging to the Ericaceae family, genus Vaccinium. V. sect. Oxycoccus, known as cranberry. It is divided into four species: V. erythrocarpum (southern mountain cranberry); V. macrocarpon (large cranberry, American cranberry, or bearberry); V. microcarpum (small cranberry); and V. oxycoccos (common cranberry or northern cranberry) [102]. The world’s most significant cranberry production is in North America, with an annual output of 436.691 tons, thus representing 58% of the world’s total cranberry production [103]. Acid soils and the peat of bogs, swamps, wet shores, and occasionally poorly drained upland meadows, are the perfect environments for growing fruits from the subgenus V. Oxycoccus, particularly these two species: V. macrocarpon and V. oxycoccos [104].
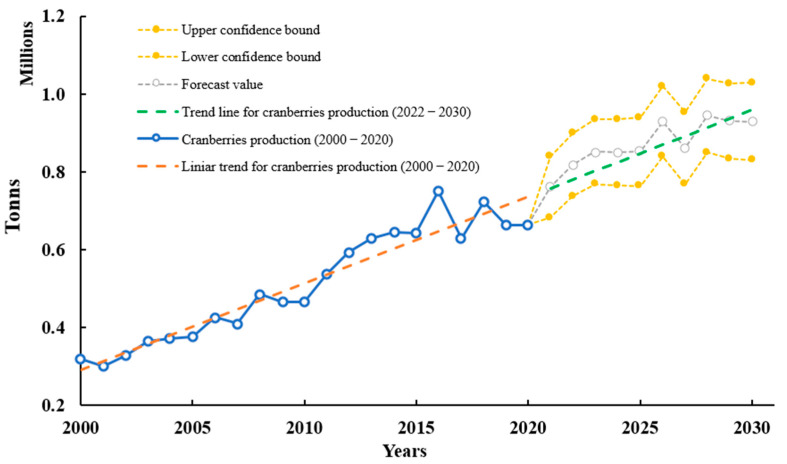
Cranberry (V. sect. Oxycoccus) is one of the most cultivated fruits in the U.S., with a global production of over 663.000 tons in 2020; this had a 108% increase from 2000 to 2020 (FAO). Based on the production and consumption of cranberry, this will also present a constant growth trend, with an increase of 44.4% (958107 tons) by 2030. In 2007, around USD 75 M was obtained from agricultural receipts [105]. In addition, considering the last 20 years of cranberry production and using Excel 2022 and FORECAST function, we predicted production until 2030 based on existing values that follow a seasonal trend. A confidence interval was estimated using interval values from 2000–2020. The interval was defined by its lower and upper bounds from 2020 until 2030. Seasonality was considered to be one decade. The confidence interval was expressed as a percentage (95%). The percentage reflects the confidence level. According to data analysis from FAO data, cranberries forecast may reach a value of 0.93 ± 0.14 M tons by 2030. Figure 2 shows a constant forecast and trendline that will increase in the following years.
Figure 2.
Evolution of world cranberry production over time. According to FAO, global cranberry production (tons) from 2000 until 2030 and TREND production until 2030. The trendline was calculated considering cranberry production every year from 2000–2020. The TREND function returns values along with a linear trend. It fits a straight line (using the least squares method) to the arrays known_ys and known_xs. TREND returns the y–values along that line for the array of new_xs that you specify.
The American cranberry (V. macrocarpon) and the European cranberry (V. oxycoccos) are the most common. The American fruit is two times larger than the European cranberry, which is between 0.6 and 1.2 cm. Cranberry juice drinks, sauce, sweetened, dried cranberries, and pemmican cranberry (a high-protein combination of crushed cranberries, dried deer meat, and melted fat) are some of the products obtained from cranberry.
3.2. Blueberries Constituents
The most important bioactive compounds in cranberries are anthocyanins, flavonols, flavan–3–ols (catechins), PACs, benzoic and phenolic acids, nonflavonoid polyphenols (phloridzin), terpenes, sterols [106], and several essential micro- (Fe, Mn, Zn, Cu, Mo, B) and macro-elements (N, P, K, Ca, Mg, S), as evidenced in Table 3 [107].
Table 3.
Content of macro- and micro-elements in V. macrocaropn and V.oxycoccos. (adapted from [107,108,109]).
| V. macrocarpon | V. oxycoccos | Method | |
|---|---|---|---|
| Macroelements, mg/100 g fw | |||
| Magnesium | 5.2–9.1 | 7.8–9.1 | AAS (Perkin Elmer AAnalyst 700, acetylene-air flame) |
| Sulfur | 5.2–14.3 | 6.5–18.2 | Turbidimetry |
| Potassium | 52.0–98.8 | 78.0–93.6 | FP (Jenwey PFP7, airpropane butane flame) |
| Calcium | 7.8–14.3 | 9.1–18.2 | AAS (Perkin Elmer AAnalyst 700, acetylene-air flame) |
| Phosphorus | 6.5–11.7 | 6.5–7.8 | Colourimetry |
| Nitrogen | 10.4–65.0 | 13.0–78.0 | Colourimetry |
| Microelements, mg/100 g fw | |||
| Iron | 0.22–1.17 | 0.33–0.42 | AAS (Perkin Elmer AAnalyst 700, acetylene-air flame) |
| Manganese | 0.06–0.57 | 2.18–3.95 | AAS (Perkin Elmer AAnalyst 700, acetylene-air flame) |
| Zinc | 0.07–0.17 | 0.14–0.19 | AAS (Perkin Elmer AAnalyst 700, acetylene-air flame) |
| Molybdenum | 0.01–0.02 | 0.01–0.02 | Colourimetry |
| Copper | 0.04–0.08 | 0.06–0.08 | AAS (Perkin Elmer AAnalyst 700, acetylene-air flame) |
| Boron | 0.03–0.12 | 0.10–0.17 | Colourimetry |
AAS—atomic absorption spectrophotometer; FP—flame photometer.
V. macrocarpon is an essential healing agent and food because of its composition, flavonoids being its major compounds among over 150 compounds, including 13 anthocyanins, 16 flavonols, and 26 phenolic acids and benzoates [108].
3.2.1. Phenolic Acids
Cranberries’ therapeutic potential, in the case of both species V. macrocarpon and V.oxycoccos, comes from their antioxidant effect, which is due to its many phytochemicals. Thus, a cranberry mixture or solution contains several classes of phenolics after the extraction process. The method used for quantification and other characterisation includes High-performance Liquid Chromatography (HPLC), Ultraviolet-Visible (UV/Vis), and Gas Chromatography (GC) [108].
In cranberry, high quantities of benzoic acid and low quantities of 2,4–dihydroxybenzoic acid, p–h–hydroxybenzoic, and o–hydroxybenzoic acids (salicylic acid) can be found. Pınar Kalın et al. found some of the main phenolic compounds in V. macrocarpon extract using the Folin–Ciocalteu method. Based on a standard gallic acid curve, they determined the quantity of total phenolics and the total phenolic content in lyophilised aqueous cranberry extract. Some of the main phenolic compounds identified were p–coumaric acid (13.0 mg/kg), p–hydroxybenzoic acid (55 mg/kg), kaempherol–3–O–glucoside (11.0 mg/kg), caffeic acid (5.0 mg/kg), and ellagic acid (3.0 mg/kg) [110,111]. The quantity of phenolic compounds depends on the cultivar and berry ripening; therefore, total phenolic acids rise to form the lowest content of 327 mg/100 g dry matter (dm) in ”Pilgrim” to the highest content of 649 mg/100 g dm in “Howes” [111]. The most present compounds in all cranberry cultivars (Pilgrim, Stevens, and Ben Lear) were caffeoyl hexoside (representing 34.6% to 54.1% of total phenolic acids) and caffeoyl dihexoside (from 16.5% to 35.0%) [112].
3.2.2. Anthocyanins and Proanthocyanidins
Anthocyanins belong to the polyphenol class and are found in most black fruits (blueberry, black currants, cranberry) and vegetables (red cabbage, radish). Studies in vivo and on experimental animals showed that anthocyanins decrease insulin resistance, protect pancreatic cells against necrosis, and increase glucose uptake by tissues in streptozotocin-induced diabetic rats and mice. Additionally, they improve insulin secretion (which can help people with type two diabetes), and particularly help women with UTIs, which are one of the most common bacterial infections [113]. Studies showed that PACs found in cranberries inhibit the adherence of p-fimbriated E. coli on the mucosal surface of the urinary tract, obstructing bacterial reproduction [114].
Anthocyanins (Table 4) give cranberries their red colour, and they are natural water-soluble pigments found in amounts 6 to 10 times higher in the peel in contrast to the pulp of the small berry (V. oxycoccos) [111]. Climate, cultivar, growing locations, and genetic traits are some of the things that influence the content of anthocyanins in cranberry. The anthocyanins level significantly increases during the maturation process in cranberry. For example, in the fruit cultivars “Pilgrim”, “Stevens”, and “Ben Lear”, anthocyanins increase by 57.3%, 47.0%, and 30.0%, respectively, from the immature stage to the mature stage [112].
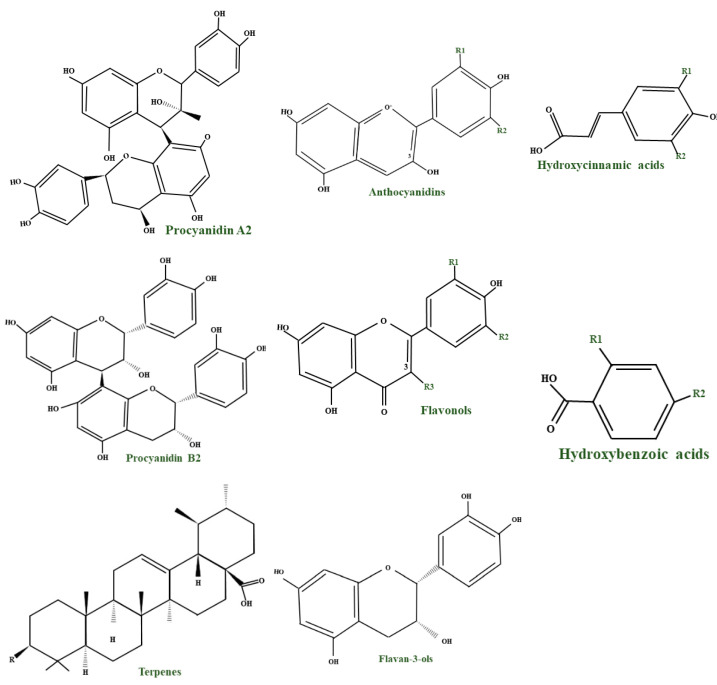
A differentiating compound in cranberry is the A-type PAC, which hinders the in vitro bond of E. coli to uroepithelial cells [111,114]. Jacques Masquelier discovered PACs in the 1940s and gave them the name ‘’vitamin P’’. An essential characteristic of PACs is their capacity to precipitate proteins and polypeptides, acting more on those with a high proline level. A-type polymers are less frequent and have at least one intermolecular bond between O7 and C2, in addition to the C–C bond (Figure 3) [102]. One of the most important constituents of PACs is epicatechin (−), whereas epicatechin, catechin (+), and (epi) gallocatechin have an important role but are present only in trace amounts [115]. Compared to V macrocarpon, which has a range of 2.80–5.05 mg/100 g fw (fresh weight), V oxycoccus has a range of 0.55–1.94 mg/100 g fw [111,116].
Figure 3.
Bioactive compounds found in cranberry; figure adapted from [115].
3.2.3. Flavonoids
Anthocyanins, flavonols, and flavan–3–ols are the elementary classes of flavonoids found in cranberry. Because of their polyphenolic structure, they play a vital role in antibacterial, antiviral, anticarcinogenic, anti-inflammatory, and vasodilatory activities, as well as in defence mechanisms and antioxidant activity. Cranberry fruit has a significant concentration of flavonoids, such as kaempferol, myricetin, and quercetin in the peel; the ripening stage determines this concentration [102,108,111].
Table 4.
| Phytonutrient | Name | Content |
|---|---|---|
| Anthocyanins | Delfinidyn derivatives * | 31.27–43.87 mg/100 g dm |
| Delfinidyn-3-O-glucoside * | 1.1–1.8 mg/100 g dm | |
| Cyanidin derivatives * | 442–967 mg/100 g dm | |
| Cyanidin-3-O-galactoside * | 119.9–180.0 mg/100 g dm | |
| Cyanidin-3-O-glucoside * | 5.5–7.3 mg/100 g dm | |
| Cyanidin-3-O-arabinoside * | 64.5–95.6 mg/100 g dm | |
| Peonidin-3-O-galactoside * | 131.3–310.3 mg/100 g dm | |
| Peonidin-3-O-arabinoside * | 42.9–95.2 mg/100 g dm | |
| Peonidin derivatives * | 192–666 mg/100 g dm | |
| Malvidin derivatives * | 29.85–58.85 mg/100 g dm | |
| Malvidin-3-O-arabinoside * | 1.4–1.9 mg/100 g dm | |
| Total anthocyanins * | 695–1716 mg/100 g dm | |
| Phenolic acid | p–Coumaric acid *** | 2–245 µg/g dw |
| p-Coumaroyl hexose * | 8.6–13.9 mg/100 g dm | |
| p-Coumaroyl hexose isomer * | 3.6–50.0 mg/100 g dm | |
| p-Coumaroyl derivatives * | 210–451 mg/100 g dm | |
| Chlorogenic acid * | 72.00–129.62 mg/100 g dm | |
| Caffeic acid *** | 5–123 µg/g dw | |
| Caffeoyl hexoside * | 92.7–190.2 mg/100 g dm | |
| Caffeoyl hexoside isomer * | 10.9–17.5 mg/100 g dm | |
| Caffeoyl and derivatives * | 39.93–68.28 mg/100 g dm | |
| Ferulic acid *** | 4–39 µg/g dw | |
| Total phenolic acid * | 327–649 mg/100 g dm | |
| Flavonols | Myricetin-3-O-galactoside * | 156.5–348.4 mg/100 g dm |
| Myricetin-3-O-glucoside * | 1.8–6.6 mg/100 g dm | |
| Myricetin-3-O-pentoside * | 6.3–55.6 mg/100 g dm | |
| Myricetin-3-O-glucoronide * | 19.0–38.5 mg/100 g dm | |
| Myricetin-arabinoside *** | 8–273 µg/g dw | |
| Sinapoyl derivatives * | 4.36–5.82 mg/100 g dm | |
| Myricetin derivatives * | 496–926 mg/100 g dm | |
| Quercetin-3-O-galactoside * | 294.6–375.8 mg/100 g dm | |
| Quercetin-3-O-pentoside * | 21.2–122.9 mg/100 g dm | |
| Quercetin-3-O-glucoside * | 4.8–11.5 mg/100 g | |
| Quercetin-p conmaroylhexoside * | 1.3–13.3 mg/100 g dm | |
| Quercetin-3-O-rhamnoside * | 6.2–13.3 mg/100 g dm | |
| Quercetin-rutinoside ***** | 12.0 mg/100 g fw | |
| Quercetin-acetyl-glucosidase ***** | 13.58 mg/100 g fw | |
| Quercetin derivatives * | 107–225 mg/100 g dm | |
| Methoxyquercetin hexoside * | 1.7–25.7 mg/100 g dm | |
| Methoxyquercetin pentoside * | 3.4–61.0 mg/100 g dm | |
| Methoxyquercetin derivatives * | 33.31–43.04 mg/100 g dm | |
| Total flavonols * | 643–1088 mg/100 g dm | |
| Flavan-3-ols and proanthocyanidins | (+)-Catechin * | 2.79–7.53 mg/100 g dm |
| (−)-Epicatechin * | 27.46–56.84 mg/100 g dm | |
| A-type PA-dimer * | 16.94–32.07 mg/100 g dm | |
| A-type PA-trimer * | 27.82–76.94 mg/100 g dm | |
| A-type PA-tetramer * | 41.51–65.61 mg/100 g dm | |
| B-type PA–dimer * | 12.62–36.75 mg/100 g dm | |
| B-type PA–trimer ****** | 0.04–2.93 mg/100 g fw | |
| Polymeric proanthocyanidins * | 651–1109 mg/100 g dm | |
| Sinapyl hexose * | 2.0–3.3 mg/100 g dm | |
| Total flavan–3–ols and proanthocyanidins * | 860–1283 mg/100 g dm | |
| Triterpenoids | Ursolic acid * | 1044–1714 mg/kg dm |
| Oleanolic acid * | 894–1137 mg/100 g dm | |
| Betulinic acid * | 635–824 mg/kg dm | |
| Sum Triterpenoids * | 2892–3671 mg/kg dm | |
| Total Sterols (β–sitosterol and stigmasterol) **** | 107.83 mg/g fw |
dm—dry matter; dw—dry weight; fm—fresh matter; fw—fresh weight; methods used to identify phytonutrients: * LC/MS Q-TOF—Liquid Chromatography/Mass Spectrometry Quadrupole Time-of-Flight; *** HPLC/ESI-MS/MS—high-performance liquid chromatography/electrospray ionization tandem mass spectrometry; **** HPLC-DAD—high-performance liquid chromatography-diode array detector; ***** HPLC-PDA—high-performance liquid chromatography/photodiode array; ****** UHPLC UV/MS—ultra-high performance ultra-violet mass spectrometer.
During this ripening, flavonol levels increased by 25%, 9%, and 1% in ’’Pilgrim’’, ’’Stevens’’, and ‘’Ben Lear’’, respectively [102,108,111]. Flavonoid content in three cranberry cultivars (Stevens, Pilgrim, Ben Lear) showed small differences. Stevens had the highest amount (142.1 mg/100 g fw), which was similar to Pilgrim (138.6 mg/100 g fw), and the lowest amount of flavonoids was found in the Ben Lear cranberry cultivar (114.2 mg/100 g fw) [119]. Fresh cranberry juice has a medium of 400 mg of total flavonoids (56%) and other phenolic compounds (44%) per litre [108].
3.2.4. Triterpenoids
Cranberry fruit is also rich in triterpenoids; they have a complex cyclic structure and exist as carboxylic acids, alcohols, or aldehydes [111,120]. The most important compounds in this class are ursolic acid, which has an anti-inflammatory effect and can inhibit liver and breast cancer. Ursolic acid’s isomers, especially cis–3–O–p–hydroxycinnamoyl ursolic acid, trans–3–O–p–hydroxycinnamoyl ursolic acid, and oleanolic acid, which are found in the wax on the peel, present anti-inflammatory, antitumor and anticancer effects. V. oxycoccus contains ursolic acid, which defends against oxidative damage and lipid oxidation [111,121]. Wu et al. (2020) identified in cranberry extract significant quantities of ursolic acid (372.97 mg/g), oleanolic acid (79.16 mg/g), and β–sitosterol and stigmasterol (107.83 mg/g) [118]. The cultivar ‘’Franklin’’ showed the highest amount of triterpenoids (3671 mg/kg dm), and the lowest content was in the “Pilgrim’’ cultivar (2892 mg/kg dm) [111].
Several studies have shown that ursolic acid can inhibit tumour colony formation in HT–29 and HCT116 human colon tumour cell lines. The growth of tumour cell lines, including HT–29 colonic cells, is inhibited by triterpenoid esters (cis– and trans–3–O–p–hydroxycinnamoyl ursolic acid) isolated from cranberry juice [118]. Another study showed that the peel samples of V. macrocarpon and V. oxycoccos contain the highest amount of the triterpenes and phytosterol compounds (11,109.86 ± 166.27 µg/g and 9582.45 ± 143.74 µg/g). Higher levels of triterpenoids and phytosterols were found in the cultivar “Stevens” (V. macrocarpon) and lower amounts in small cranberry (V. oxycoccos). The ursolic acid found in fruit samples was 64.80% for the large cranberries and 62.64% for the small cranberries [121].
3.3. Antimicrobial Effect
Four American cranberry (V. macrocarpon) cultivars, ‘’Stevens’’, ‘’Pilgrim’’, ‘’Ben Lear’’, and ‘‘Black Veil’’, were grown in the same experimental field of Kaunas Botanical Garden of Vytautas Magnus University and analysed.
The antimicrobial activity was performed on Gram-positive Listeria monocytogenes (ATCC 19117), Bacillus cereus (ATCC 10876), B. subtilis (ATCC 6633), Micrococcus luteus (ATCC 9341), Enterococcus faecalis (ATCC 29212), and S. aureus (ATCC 25923), and Gram-negative E. coli (ATCC25922), Enterobacter aerogenes (ATCC 13048), Salmonella typhimurium (ATCC 14028), and Slm. agona bacteria. Clavulanic acid was used at 30/10 μg/mL for positive control and acidified ethanol for negative control. The agar well diffusion method was used to test the antimicrobial effect. All extracts exposed antimicrobial properties, and the most effective was Pilgrim. This method showed that B. cereus and M. luteus were the most sensitive, with an inhibition zone between 2.28 and 2.24 cm. All test cultures were sensitive to the clavulanic acid 30/10 μg sensi-disc, omitting B. cereus, which was more responsive to berry extract. Additionally, eight strains of yeast isolated from dairy products, industrial air, and equipment washing water were used: Debaryomyces hansenii, Trichosporon cutaneum, Kluyveromyces marxianus var. lactis, Saccharomyces cerevisiae, Candida parapsilosis, Torulaspora delbrueckii, Pichia kluyveri, and Rhodotorula rubra. Cranberry extracts did not affect the multiplication of any of the eight yeast species [122,123].
In addition, cranberries have an essential effect on the inhibition of oral pathogenic bacteria, such as Streptococcus mutans, Streptococcus gordonii, Streptococcus sobrinus, Actinomyces naeslundii, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, and Enterococcus faecalis. The method used to prove the antimicrobial effect on oral pathogenic bacteria was the agar diffusion assay. In addition, anthocyane and proanthocyanidin found and extracted from cranberries were antibacterial, but S. mutants was not supported by cranberry extract, inhibiting E. faecalis partially. For positive control, chlorhexidine (CHX) 2% was used, and this study revealed that antibacterial efficiency is influenced by incubation time and concentration. Cranberry juice at 90% concentration and exposure times of 60 s could suppress four out of eight pathogenic species (S. sobrinus, F. nucleatum, A. actinomycetemcomitans, and P. gingivalis) [124].
3.4. Medicinal Effect of Cranberry Consumption
Cranberries have many medicinal applications, and their consumption can prevent many diseases (Table 5), such as urinary tract inflammation, cystitis, oxidative stress, cardiovascular diseases, obesity, type 2 diabetes, microbial infections, tooth decay, periodontitis, and cancers [111].
Table 5.
Prevention of different types of diseases with cranberry consumption and mechanisms.
| Type of Disease | Mechanism | References |
|---|---|---|
| Gastric cancer | A-type procyanidins in cranberry ↓ the adhesion of H. pylori on human gastric mucus and have an inhibitory effect | [125,126] |
| Obesity | Cranberry ↓ lipid accretion by lowering mRNA level of some genes associated with fatty acid-binding protein, lipoprotein lipase, fatty acid synthase, hormone-sensitive lipase, and perilipin | [127,128,129] |
| Type 2 diabetes | Quercitin ↓ gastric assimilation of glucose and with myricetin can ↓ glucose transporter type 4 mediated glucose assimilation | [130,131] |
| Urinary tract inflammation | A-type PACs ↓ adhesion of P-fimbriated uropathogenic E. coli to uroepithelial cells | [114,116,132] |
| Periodontitis | Cranberry non-dialysable material ↓ proliferation of P.gingivalis, T.forsythia, and T.denticola in periodontal pockets A-type cranberry PACs ↓ production of metalloproteinases |
[124,133,134] |
| Anti-inflammatory | Quercetin ↓ of the nuclear factor-kappa B (NF-κB) pathway, NDM lower lipopolysaccharide-induced inflammatory cytokine production | [135] |
| Nonalcoholic fatty liver disease | In vitro study > cranberry diet 3 months > alanine reduction aminotransferase and insulin, + lipid profile effect, insulin resistance and hepatic steatosis in NAFLD patients | [136] |
| Microbial | Cranberry extracts ↓ on pathogenic bacteria: Listeria monocytogenes (ATCC 19117), B. cereus (ATCC 10876), B. subtilis (ATCC 6633), M. luteus (ATCC 9341), E. faecalis (ATCC 29212), S. aureus (ATCC 25923) and Gram-negative E. coli (ATCC25922), Enterobacter aerogenes (ATCC 13048), Slm. typhimurium (ATCC 14028), and Slm. agona bacteria | [122,123] |
| Cardiovascular | The expression of inflammatory genes suited for cardiovascular diseases is ↓ by resveratrol (polyphenol present in cranberry juice) by inflecting the NF-kB and JAK STAT3 pathways in cultured cells | [137,138] |
| Breast and colon cancer | Quercitin and proanthocyanidin inhibited the expansion of MCF-7 human breast adenocarcinoma and HT–29 human colon adenocarcinoma | [106,118] |
| Leukaemia and lung cancer | Ursolic acid can inhibit the growth of some leukaemia cell lines and A-549 human lung carcinoma | [106,139] |
Note: JAK—(Janus kinase inhibitor); NF-kb—Nuclear factor kappa-light-chain-enhancer of activated B cells; STAT3—signal transducer and activator of transcription 3; PACs—proanthocyanidins.
Gastric cancer: is one of the most frequent types of cancer for all sexes and ages. According to the World Health Organization (WHO) [125], H. pylori are often linked with the evolution of most gastric ulcers. However, eradicating antibiotics is not a strategy because H. pylori colonisation can show some health benefits in reliable situations. Cranberry has many bioactive compounds, including PACs with A–type double linkages. In addition, the presents of the PACs that have an anti-adhesion effect on bacteria are important because they inhibit the initial stage of the infection instead of killing the bacteria [125]. To demonstrate the anti-adhesion effect, Burger et al. (2000) conducted a study on the adhesion of three strains of H. pylori (BZMC-25, EHL-65, and 17874) on gastric mucus acquired from a stomach taken after post-mortem surgeries. Non-dialysable material (NDM) was a cranberry juice dialysed against distilled water in dialysis bags; after six days of dialysis, NDM was lyophilised. NDM at 100 μg mL−1 concentrations inhibited H. pylori BZMC-25 and constrained adhesion to human gastric mucus. The inhibitory effect was dose-dependent, and 50% of the inhibitory concentration relied on BZMC-25, EHL-65, and 17,874 strains [126].
Obesity: many studies show that cranberry reduces lipid accretion by lowering the mRNA level of some genes associated with fatty-acid-binding protein, lipoprotein lipase, fatty acid synthase, hormone-sensitive lipase, and perilipin 1. However, one of the most important targets for obesity prevention is to decrease leptin and increase adiponectin gene expression and adipocytokines secretion A [127]. In vivo studies on obese diabetic mice have shown that a dose of 5% and 10% of cranberry powder in the diet for six weeks and a cranberry extract (200 mg/kg) for eight weeks increased HDL-cholesterol level and decreased insulin and glucose level. The decreased hepatic, intestinal, and plasma triglyceride accumulation reduced oxidative stress and inflammation [128]. In a study on mice, Kunkel et al. (2012) showed that ursolic acid identified in cranberry reduces obesity, improves glucose tolerance, and decreases hepatic steatosis [129].
Diabetes: cranberries are rich in phenolic compounds such as quercetin which can inhibit gastric assimilation of glucose in the porcine model [130]. Besides quercetin, they also contain myricetin, which may inhibit glucose transporter type 4 mediated glucose assimilation by rat adipocytes [140]. Patients with type 2 diabetes were involved in two 12-week studies to prove that cranberry bioactive constituents help features of metabolic syndrome and diabetes. Cranberry juice significantly reduces restrained glucose, while cranberry extracts mark down total and LDL–cholesterol. Some studies show that dried low-calorie cranberry-enriched high-fat meal challenge induced postprandial glycaemia, inflammation, and lipid peroxidation in diabetes [131]. Another study shows that daily consumption for 12 weeks of cranberry juice (240 mL) and blueberry extract (9.1–9.8 mg of anthocyanins) for 8 to 12 weeks improved glucose control in type 2 diabetes subjects [141].
Urinary tract infection (UTI): is a bacterial infection which affects young and sexually active women. Approximately 1 in 3 women will experience an episode of UTI, requiring antibiotics [114]. In vitro studies show that A-type PACs constrain the adhesion of P-fimbriated uropathogenic E. coli to uroepithelial cells [116]. Antibiotics to treat UTIs have been claimed to be very functional, but these antibiotics can damage intestinal microbiota and cause resistance among uropathogens. PACs are usually catabolised by the colon microbiota to give bioactive phenolic metabolites. This phenolic metabolite may be the responsible compound behind the anti-adhesion effect. D–mannose also inhibits adhesion on uroepithelial cells in vitro. Another helpful characteristic of cranberry juice is the pH of 2.5, which causes changes in urine’s physical properties (acidification) [132].
Periodontitis: is an inflammatory disease which affects tooth-supporting tissues (periodontal ligament and alveolar bone) and is induced by Gram-negative anaerobic bacteria. NDM can inhibit the proliferation of P.gingivalis, T.forsythia, and T.denticola in periodontal pockets. Unusual production of cytokines by host cells caused by periodontopathogens damage tooth-supporting tissues; studies have demonstrated that cranberry was an excellent inhibitor of pro-inflammatory cytokine and chemokine replies caused by lipopolysaccharides [124,133,134].
4. Another Vaccinium sect. V. membranaceum
4.1. History, Nomenclature, and Production
Approximately 400 species in the diverse Vaccinium genus are dispersed quasi-globally, with Antarctica and Australia as the exceptions. The genus is commonly described as shrubs or perennial vines with fleshy and moderate-sized, usually edible fruit [142,143]. Berries are considered sources of many nutrients, such as minerals, vitamins, sugars, fibre, and other compounds, with health benefits that may vary with the stress plants face [144]. Based on the taxonomical interpretation, up to 14 Vaccinium species are described in the Pacific Northwest; some examples are defined in Table 6. These species bear fruits which are often referred to as huckleberries; the same can be said about the more Eastern species, yet they are very much distinct from “true huckleberries”, which are found in the Gaylussacia genus [145].
Huckleberries are ericaceous (Ericaceae or Heath family) tetraploid (2n = 48) shrubs with fleshy, flavorfanthocyanicyan-rich, edible berries [146]. Western huckleberries, known as bilberries, mountain huckleberries, gooseberries, big huckleberries, black huckleberries, whortleberries, and thin-leaf huckleberries, are found in the genus Vaccinium section Myrtillus and are related to sect. Cyanococcus highbush (V. corymbosum L.) and lowbush blueberries (V. angustifolium Ait.). This section will focus on huckleberry species, primarily addressing the V. membranaceum plant.
The human usage of huckleberries may extend to as far back as 11.000 B.P. (Before Present). They have had significant importance in the cultural and socio-economical life of native peoples. They were eaten raw, cooked, mashed, and dried in the sun as cakes. In the 1800s, berry picking started to grow to an industrial level, taking advantage of the invention of cans, and by the 1920s, commercial companies had been established. In 1932, huckleberries were a substantial crop, with 1 M.T. picked in Montana and marginally less in Washington State. Around this time, an interest in better understanding huckleberries led to Schultz’s chromosome description (2n = 48) in 1944. In the decades that followed, the compositional aspect of these berries became better understood by Kuhlein in 1989, and their list of nutrient values in 2004 by Lee, Finn, and Wrolstad, with research on anthocyanin pigment and phenolic compounds [143,147,148,149].
Table 6.
| V. membranaceum | V. parvifolium | V. scoparium | V. ovalifolium | V. ovatum | V. caespitosum | V. deliciosum | |
|---|---|---|---|---|---|---|---|
| Names | bilberries, mountain huckleberry, grouseberries, hortleberries, black huckleberry, hortleberries, and thin-leaf huckleberry | red huckleberry, red bilberry | small-leaved huckleberry, grouseberry, dwarf red whortleberry, and red alpine blueberry | oval-leaf blueberry, oval-leaf bilberry, oval-leaf huckleberry, Alaska blueberry | evergreen huckleberry | dwarf blueberry, dwarf bilberry, dwarf huckleberry, and dwarf whortleberry | cascade huckleberry, cascade bilberry or blue huckleberry |
| Flower | creamy-pink | greenish to pinkish | pink | pink | bright pink | pink | pink |
| Fruit/Berries | large, shining black, dull black, deep purple, rarely red, 9–13 mm d. | red, occasionally faintly glaucous, 7–9 mm diam, more tart than sweet | translucent red, red, or bluish purple, 4 -6 mm d., soft, tart | bright blue, glaucous, dull purplish black or black, 810 mm d. | purplish-black, 6–9 mm d. | bright blue and glaucous, rarely dull black, 59 mm d., great flavour | blue and glaucous, occasionally dull black, maroon, or red, 9–15 mm d., especially flavorful berries |
| Distribution | Rocky Mountains from SW-NW Territories S to N California and N Utah | Pacific coast of N America from Alaska to N California, inland to SE British Columbia | SE British Columbia and adjacent Alberta, E to the Black Hills of South Dakota, and south to SW Colorado | Pacific Rim from Central Japan, Kamchatka, Aleutian Islands, S along the Pacific coast to S central Oregon and inland to N Idaho | Pacific Coast from British Columbia to central California | Alaska to Newfoundland, southward along the Atlantic Seaboard to S Maine and S Vermont; in the W, S to the W highlands of Guatemala | Pacific coastal mountain ranges from S British Columbia to N California, Montana and Idaho |
| Commercial value | food industry: jams, syrups, tea, culinary uses; medicinal uses; cosmetic uses | berries—food industry: jam, jelly, wine; leaves—medicinal use, ornamental use | culinary use | culinary use | the food industry, such as jam; medicinal use; ornamental use | the food industry; ornamental use | food industry |
| * TP | 1.70 mg of GAE/g of FW | 0.81 mg of GAE/g of FW | - | 2.81 mg of GAE/g of FW | 2.84 mg of GAE/g of FW | - | 1.41 mg of GAE/g of FW |
| ** TA | 1.69 mg of C3G/g of FW | 0.11 mg of C3G/g of FW | - | 3.07 mg of C3G/g of FW | 3.64 mg of C3G/g of FW | - | 1.35 mg of C3G/g of FW |
| *** AC | ORAC 21 µmol of TE/g of FW, FRAP 40.5 µmol of TE/g of FW | ORAC 7.3 µmol of TE/g of FW FRAP 10 µmol of TE/g of FW | - | ORAC 37.8 µmol of TE/g of FW FRAP 76.2 µmol of TE/g of FW | ORAC 41.1 µmol of TE/g of FW—FRAP 70.2 µmol of TE/g of FW | - | ORAC 14.6 µmol of TE/g of FW FRAP 30.2 µmol of TE/g of FW |
| **** F3 and F | Catechins > 240 µg/g of FW | Catechins > 154 µg/g of FW | - | Catechins > 104 µg/g of FW | Catechins > 69 µg/g of FW | - | Catechins > 109 µg/g of FW |
| Chlorogenic acid | 62.6 µg/g of FW | 60.2 µg/g of FW | - | 1< µg/g of FW | 466 µg/g of FW | - | 72.4 µg/g of FW |
| Caffeic acid | 17.6 µg/g of FW | 150 µg/g of FW | - | 5.1 µg/g of FW | 5.8 µg/g of FW | - | 41.3 µg/g of FW |
| Ferulic acid | 21.7 µg/g of FW | 38.5 µg/g | - | 17.9 µg/g of FW | 109 µg/g of FW | - | 22.6 µg/g of FW |
| p-hydroxybenzoic acid | 1.5 µg/g of FW | 553 µg/g of FW | - | 1.6 µg/g of FW | 12.1 µg/g of FW | - | 6.9 µg/g of FW |
| p-coumaric acid | 21.1 µg/g of FW | 97.3 µg/g of FW | - | 23.9 µg/g of FW | 32.4 µg/g of FW | - | 16.6 µg/g of FW |
| ***** TPC | 124.5 µg/g of FW | 899 µg/g of FW | - | 48.5 µg/g of FW | 625.3 µg/g of FW | - | 159.8 µg/g of FW |
| ****** Anthocyanidins | 1294 µg/g of FW | 112.9 µg/g of FW | - | 2179 µg/g of FW | 1850 µg/g of FW | - | 996.3 µg/g of FW |
FW—fresh weight; d—diameter; * TP—total phenolics; ** TA—total anthocyanins; *** AC—antioxidant capacity; **** F3 and F—Flavan-3-ol and Flavonol content; ***** TPC—Total phenolic acid content; ****** Anthocyanidins (cyanidin, delphinidin, malvidin, peonidin, and petunidin); ORAC—oxygen radical absorbance capacity.
Their indigenous distribution ranges chiefly in the western part of the North American continent, in the Rocky Mountains from the southwestern part of the Northwest Territories, northern Utah, and northern California, with disjunct populations in Michigan and Ontario to the east [152,153]. In Montana, the plant can also be found with the name globe huckleberry, with some plants regarded as V. globular in the Rocky Mountains. It distinguishes itself with remarkable ecological plasticity, reflected in the wide altitude range at which it can be found [148,150,151,153,154].
V. membranaceum is an upright, deciduous shrub standing from 0.3 to 2 m, forming small to vast clumps and seldom crown-forming. The fruit is spherical, about 1.5 cm in diameter, and sweet, with colours ranging from black to purple to red and, very rarely, white. Huckleberries require acidic and, usually but not imperatively, moist soils to become established, V. membranaceum being known for its difficulty in establishment.
4.2. Constituents
Mountain huckleberry is rich in aroma and flavour chemicals. Anthocyanins and polyphenolics are secondary plant metabolites. They are a varied group, with over 4000 flavonoids having been described. These compounds are essential to food quality through taste, appearance, and health benefits.
Through Lee’s et al. 2004 research, constituent nutrients of the V. membranaceum belonging to this group have been calculated. First, compound extraction (acetone and 70% aqueous acetone) occurred, after which the samples were partitioned (1:2 acetone chloroform). After evaporation and distillation, the samples were HPLC-filtered. This resulted in a pH of 2.6, 12.7 °Brix, total monomeric anthocyanin content of 167 mg of cyanidin 3-glucoside/100 g of frozen berries, and phenolic content of 617 mg of gallic acid/100 g of frozen berries. The major polyphenolics in V. membranaceum were neochlorogenic acid (3–O–caffeoylquinic acid, 26% of the total peak area measured at 320 nm) and a cinnamic acid derivative (peak 2, 21% of the total peak area measured at 320 nm). Minor polyphenolics were identified in the ethyl acetate fraction, such as protocatechuic acid, gallic acid, and epicatechin [155].
In another 2004 study by Lee et al., which analysed fresh huckleberry fruits, total monomeric anthocyanin content with a range of 101 to 360 mg/100 g and a content of total phenols membranaceum ranging from 367 to 1286 mg/100 g [145]. The first nutritional report of V. membranaceum dates from Kuhlein’s analysis of fresh berries in 1989. For 100 g, this fruit quantified moisture content of 86%, 0.6% protein, 0.5% fat, 13.1% carbs, 2% fibre, and 54 kcal. For minerals: 14 mg Ca, 17 mg P, 0.4 Na, 8 mg Mg, 0.2 mg Fe, 0.1 mg Zn, 0.1 mg Cu, 2.5 mg Mn, and 0.3 mg Sr. The most important vitamin is represented by 6.6 mg of ascorbate and 0.5 carotenes [156].
The 2004 study by Taruscio et al. highlighted the presence and content of flavonoids and phenolic acids and the antioxidant capacity of V. membranaceum. Thus, the total phenolics were found at 1.70 mg of GAE (gallic acid equivalents)/g of FW (fresh-frozen weight); total anthocyanins at 1.69 mg of C3G(cyanidin 3-glucoside)/g of FW; flavan–3–ol and flavonol content were measured for the catechins (catechin and epicatechin) level at over 240 µg/g of FW; the phenolic acids measurements: chlorogenic acid 62.6 µg/g of FW; caffeic acid 17.6 µg/g of FW; ferulic acid 21.7 µg/g of FW; p–hydroxybenzoic acid 1.5 µg/g of FW; p–coumaric acid 21.1 µg/g of FW; anthocyanidins at 1294 µg/g of FW; and with an antioxidant capacity at ORAC (oxygen radical absorbance capacity) 14.6 µmol of TE (Trolox equivalents)/g of FW and FRAP (ferric reducing ability of plasma) 30.2 µmol of TE/g of FW [149].
4.3. Ecological Value, Commerce, and Economy
Huckleberries have been important culturally and socioeconomically to the Native American population and an important food source for wild fauna. They represent forage for black and grizzly bears, with studies suggesting that these berries represent up to 10% of their diet, with range overlap between the two beings also observed, corroborating the indispensability of huckleberries from this bear’s diet and preparation for hibernation. Deer, elk, moose, foxes, small mammals, gulls, turkeys, and other birds consume parts of the shrub. Pollinators also benefit from this plant’s flowers; namely bees and bumblebees [148,154,157,158].
V. membranaceum is considered to have the best taste of all Western huckleberries and has a long, thousand-year-old harvesting history for personal and commercial use [146]. Even in modern times, the berries are harvested from wild, naturally occurring stands, such as fields and open-canopy, with the majority belonging to public U.S. lands [152].
The huckleberries have had an essential economic aspect in North America since becoming popular with the European colonists. Besides being a food source, they were used to obtain natural dyes or smoking mixtures. In the early 2000s, there was a sudden increase in demand. V. membranaceum has intense, easily identifiable flavours that fit into market niches. The berries are picked from wild stands between July and August and are eaten fresh, baked in pies, pancakes, syrups, candies, vinaigrettes and salad dressings, soft drinks and ales, made into jellies and jams, canned, or frozen. From huckleberry leaves, tea can also be produced. Likewise, there are shampoos, lotions, soaps, and other products made out of huckleberries. To match the demand, the University of Idaho has tried to produce cultivars for field cultivation and managed forest systems. In 2013, huckleberry products sales made over USD 1 M in revenue for Montana, with jellies, jams and preserves representing over 40% of the production. A big challenge for the commercial expansion of this fruit is characterized by the difficulty of its endemic environment, rough mountain sites, and lack of accessible infrastructure [142,143,146,148,151,152].
5. Economic Aspects of Using Waste and by-Products of Berries
The management of natural resources relies on the ecological consciousness of species and populations, along with the ethnic and social-economic features of a particular area [159,160]. Modern society has an ever-increasing requirement for using aromatic and medicinal natural resources [161,162]. The leaves are generally considered a by-product of berry-fruit production, even though they contain important content of phenolic compounds. Booth et al. (2012) analysed the cranberry leaf extract from a safety point of view. They found that the leaf is safe for consumption and is mainly composed of organic acids, simple sugars, catechins, flavonoids, fatty acid glycosides, proanthocyanidins, rosarin, and iridoids [163].
Meanwhile, bilberry leaves are primarily collected between May and June, throughout blooming, and they are mainly used as a traditional remedy. Conventionally, they are used particularly due to their anti-inflammatory, tonic, antiseptic, and astringent properties. They are also efficient in the treatment of UTIs, liver diseases, bladder stones, rheumatism, and other disorders [164]. A recent review article analysed the phenolic compounds of five Vaccinium species, namely blueberry, bilberry, bog bilberry, bearberry, and lingonberry. As concluded, only some studies have examined these aspects, even though they have numerous bioactive compounds [19]. The main phytochemicals identified within the leaves are arbutin, quercetin, and chlorogenic acid. Arbutin and its derivatives increase antimicrobial activity against Gram-negative bacteria (Proteus vulgaris, E.coli, H. pylori) [165]. Quercetin is efficient in avoiding multiple chronic diseases and can be mostly found in fruits with red leaves, along with kaempferol, proanthocyanidins, and hydroxycinnamic acids [166]. A high content of soluble phenolics are located in the leaves of these species, such as chlorogenic acid (55%) and flavonol glycosides (30%) [167].
From the fruits and leaves of berries, herbal tea can be produced, which displays essential antioxidant and antibacterial activity. The administration of these berry juices can hinder the development of atherosclerosis. After 12 weeks of consumption, the anthocyanins in these juices inhibited the aortic lipid accumulations between 79–96%, activated a diminished movement of hepatic antioxidant enzymes, and facilitated neuronal behaviour and functions [168]. Along with atherosclerosis, these compounds have also been applied to alleviate throat, mouth, and other infections, nausea, stomatitis, aphtha, diarrhoea, diabetes, inflammations, dysentery, and various cancer types [169,170].
Another by-product that is usually generated through the extraction of bioactive compounds for the production of pharmaceutical formulations is berry pomace. These by-products are still full of valuable compounds, bioactive phytochemicals and nutrients. To reduce these “wastes”, the extraction should be effectuated with food-grade solvents. Afterwards, as a recent study demonstrated, good functional ingredients can be formulated from these by-products with multistep high-pressure fractionation [171].
As several studies highlight, using the by-products of berry fruit production presents an essential economic aspect through the reduction of wastes and valorification of them within functional food products, nutraceuticals, or supplements. These wastes are also rich or even richer in phenolic compounds, flavonoids, and anthocyanins than fruits.
6. Conclusions and Perspectives
Parts of Vaccinium species, such as leaves, stems, and especially fruits, are widely utilized in functional food, in various traditional medicinal products, cosmetics, and even in ornamental product generation. Their abundant bioactive compounds, antidiabetic, anti-inflammatory, anti-carcinogenic, antioxidant, and other health-related effects, are extensively used to treat multiple diseases.
The Vaccinium genus found worldwide, especially those belonging to cranberry, blueberry (bilberry), and huckleberry species, have been analysed regarding their distribution, history, uses, health benefits, and chemical profile. As can be observed, the huckleberry species still needs some further studies into applicability.
Additionally, an important aspect should be the focus on the by-products, such as the leaves of most cultured berries worldwide. The bioavailability of the identified polyphenols and their mechanism of action should be better investigated. Additionally, the integration of bioactive compounds extracted from leaves and their introduction in nutraceuticals or functional foods should be further evaluated.
Acknowledgments
The authors would like to thank their colleagues from the Department of Food Science for their continued support.
Author Contributions
Conceptualization, G.A.M. and D.C.V.; methodology, E.S.; software, D.C.V.; validation, G.A.M. and T.B.-E.; formal analysis, D.C.V.; resources, G.A.M., R.O., M.B., D.A.S. and T.B.-E.; writing—original draft preparation, G.A.M. and T.B.-E.; writing—review and editing, D.C.V.; visualisation, G.A.M. and T.B.-E.; supervision, G.A.M.; project administration, D.C.V.; funding acquisition, D.C.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 101007783—FRIETS and “The development of advanced and applied research skills in the logic of STEAM+ Health”, POCU/993/6/13/153310, project co-financed by the European Social Fund through the Human Capital Operational Program 2014-2020.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.FAO Data Food and Agriculture Data (Datenbank) [(accessed on 14 December 2022)]. Available online: https://www.fao.org/faostat/en/#data.
- 2.Coman V., Teleky B.-E., Mitrea L., Martău G.A., Szabo K., Călinoiu L.-F., Vodnar D.C. Advances in Food and Nutrition Research. Volume 91. Academic Press; Cambridge, MA, USA: 2019. Bioactive potential of fruit and egetable wastes; pp. 157–225. [DOI] [PubMed] [Google Scholar]
- 3.Precup G., Pocol C.B., Teleky B.-E., Vodnar D.C. Awareness, Knowledge, and Interest about Prebiotics—A Study among Romanian Consumers. Int. J. Environ. Res. Public Health. 2022;19:1208. doi: 10.3390/ijerph19031208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Szabo K., Mitrea L., Călinoiu L.F., Teleky B.-E., Adria G., Plamada D., Pascuta M.S., Nemeş S.-A., Varvara R., Vodnar D.C. Natural Polyphenols Recovery from Apple-, Cereal-, and To- mato- Processing By-Products, and Related Health-Promoting Properties. Molecules. 2022;22:7977. doi: 10.3390/molecules27227977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martau G.A., Teleky B.-E., Ranga F., Pop I.D., Vodnar D.C. Apple Pomace as a Sustainable Substrate in Sourdough Fermentation. Front. Microbiol. 2021;12:3850. doi: 10.3389/fmicb.2021.742020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teleky B.-E., Mitrea L., Plamada D., Nemes S.A., Călinoiu L.-F., Pascuta M.S., Varvara R.-A., Szabo K., Vajda P., Szekely C., et al. Development of Pectin and Poly(vinyl alcohol)-Based Active Packaging Enriched with Itaconic Acid and Apple Pomace-Derived Antioxidants. Antioxidants. 2022;11:1729. doi: 10.3390/antiox11091729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zia S., Khan M.R., Shabbir M.A., Aadil R.M. An update on functional, nutraceutical and industrial applications of watermelon by-products: A comprehensive review. Trends Food Sci. Technol. 2021;114:275–291. doi: 10.1016/j.tifs.2021.05.039. [DOI] [Google Scholar]
- 8.Precup G., Teleky B.-E., Ranga F., Vodnar D.C. Assessment of Physicochemical and Rheological Properties of Xylo-Oligosaccharides and Glucose-Enriched Doughs Fermented with BB-12. Biology. 2022;11:10836. doi: 10.3390/biology11040553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teleky B.E., Martău G.A., Ranga F., Pop I.D., Vodnar D.C. Biofunctional soy-based sourdough for improved rheological properties during storage. Sci. Rep. 2022;12:17535. doi: 10.1038/s41598-022-22551-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Precup G., Mitrea L., Nemes A., Călinoiu L.-F., Martău G.-A., Teleky B.E., Coman V., Vodnar D.C. Gastronomy and Food Science. Academic Press; Cambridge, MA, USA: 2021. Food processing by-products and molecular gastronomy; pp. 137–164. [Google Scholar]
- 11.Martău G.A., Coman V., Vodnar D.C. Recent advances in the biotechnological production of erythritol and mannitol. Crit. Rev. Biotechnol. 2020;40:608–622. doi: 10.1080/07388551.2020.1751057. [DOI] [PubMed] [Google Scholar]
- 12.Tundis R., Tenuta M.C., Loizzo M.R., Bonesi M., Finetti F., Trabalzini L., Deguin B. Vaccinium species (Ericaceae): From chemical composition to bio-functional activities. Appl. Sci. 2021;11:5655. doi: 10.3390/app11125655. [DOI] [Google Scholar]
- 13.Wrońska-Pilarek D., Szkudlarz P., Bocianowski J. Systematic importance of morphological features of pollen grains of species from Erica (Ericaceae) genus. PLoS ONE. 2018;13:e0204557. doi: 10.1371/journal.pone.0204557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christenhusz M.J.M., Byng J.W. The number of known plants species in the world and its annual increase. Phytotaxa. 2016;261:201–217. doi: 10.11646/phytotaxa.261.3.1. [DOI] [Google Scholar]
- 15.Gailīte A., Gaile A., Ruņģis D.E. Genetic diversity and structure of wild vaccinium populations-v. Myrtillus, v. vitis-idaea and v. uliginosum in the Baltic states. Silva Fenn. 2020;54:10396. doi: 10.14214/sf.10396. [DOI] [Google Scholar]
- 16.Kulkarni K.P., Vorsa N., Natarajan P., Elavarthi S., Iorizzo M., Reddy U.K., Melmaiee K. Admixture analysis using genotyping-by-sequencing reveals genetic relatedness and parental lineage distribution in highbush blueberry genotypes and cross derivatives. Int. J. Mol. Sci. 2021;22:163. doi: 10.3390/ijms22010163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Role H.E., Soybeanfighting O.F., Hunger W., Thoenes P. FAO Commodities and Trade Division, Basic Foodstuffs Service 1, 2. Plant Prod. 2004:1–32. [Google Scholar]
- 18.Stefănescu B.-E., Călinoiu L.F., Ranga F., Fetea F., Mocan A., Vodnar D.C., Crisan G. The Chemical and Biological Profiles of Leaves from Commercial Blueberry Varieties. Plants. 2020;9:1193. doi: 10.3390/plants9091193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ştefanescu B.E., Szabo K., Mocan A., Crisan G. Phenolic compounds from five ericaceae species leaves and their related bioavailability and health benefits. Molecules. 2019;24:2046. doi: 10.3390/molecules24112046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clapa D., Nemeș S.-A., Ranga F., Hârța M., Vodnar D.-C., Călinoiu L.-F. Micropropagation of Vaccinium corymbosum L.: An Alternative Procedure for the Production of Secondary Metabolites. Horticulturae. 2022;8:480. doi: 10.3390/horticulturae8060480. [DOI] [Google Scholar]
- 21.Mitrea L., Nemes S.-A., Szabo K., Teleky B.-E., Vodnar D.-C. Guts Imbalance Imbalances the Brain: A Review of Gut Microbiota Association With Neurological and Psychiatric Disorders. Front. Med. 2022;9:813204. doi: 10.3389/fmed.2022.813204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aaby K., Grimmer S., Holtung L. Extraction of phenolic compounds from bilberry (Vaccinium myrtillus L.) press residue: Effects on phenolic composition and cell proliferation. Lwt. 2013;54:257–264. doi: 10.1016/j.lwt.2013.05.031. [DOI] [Google Scholar]
- 23.Plamada D., Vodnar D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients. 2022;14:137. doi: 10.3390/nu14010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vodnar D.C., Călinoiu L.F., Mitrea L., Precup G., Bindea M., Păcurar A.M., Szabo K., Ştefănescu B.E. Functional and Medicinal Beverages. Academic Press; Cambridge, MA, USA: 2019. A.M.; Szabo, K.; Ştefănescu, B.E. A New Generation of Probiotic Functional Beverages Using Bioactive Compounds From Agro-Industrial Waste. [Google Scholar]
- 25.Vodnar D.C., Calinoiu L.F., Mitrea L. Editorial: Exploiting the effect of dietary fibre on the gut microbiota in patients with pelvic radiotherapy. Br. J. Cancer. 2022;127:1575–1576. doi: 10.1038/s41416-022-01993-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickerson F., Severance E., Yolken R. The microbiome, immunity, and schizophrenia and bipolar disorder. Brain. Behav. Immun. 2017;62:46–52. doi: 10.1016/j.bbi.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pascuta M.S., Vodnar D.C. Nanocarriers for sustainable active packaging: An overview during and post COVID-19. Coatings. 2022;12:102. doi: 10.3390/coatings12010102. [DOI] [Google Scholar]
- 28.Martău G.A., Călinoiu L.F., Vodnar D.C. Bio-vanillin: Towards a sustainable industrial production. Trends Food Sci. Technol. 2021;109:579–592. doi: 10.1016/j.tifs.2021.01.059. [DOI] [Google Scholar]
- 29.Vodnar D.C., Teleky B.-E. Recent Trends in Antibacterial Coatings and Biofilm. Coatings. 2023;13:255. doi: 10.3390/coatings13020255. [DOI] [Google Scholar]
- 30.Pascuta M.S., Varvara R., Teleky B.-E., Szabo K., Plamada D., Nemes S.-A., Mitrea L., Mărtau G.A., Ciont C., Călinoiu L.-F., et al. Polysaccharide-Based Edible Gels as Functional Ingredients:Characterization, Applicability, and Human Health Benefit. Gels. 2022;8:524. doi: 10.3390/gels8080524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braic R.Ş., Vari C., Imre S., Huţanu A., Fogarasi E., Todea T., Groşan A., Eşianu S., Laczkó-Zöld E., Dogaru M. Vaccinium extracts as modulators in experimental type 1 diabetes. J. Med. Food. 2018;21:1106–1112. doi: 10.1089/jmf.2017.0141. [DOI] [PubMed] [Google Scholar]
- 32.Bujor O.C., Tanase C., Popa M.E. Phenolic antioxidants in aerial parts of wild Vaccinium species: Towards pharmaceutical and biological properties. Antioxidants. 2019;8:649. doi: 10.3390/antiox8120649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dulf E., Vodnar D.C., Danku A., Martau G.A., Teleky B.-E., Dulf F.V., Ramadan M.F., Crisan O. Mathematical Modeling and Optimization of Lactobacillus Species Single and Co-Culture Fermentation Processes in Wheat and Soy Dough Mixtures. Front. Bioeng. Biotechnol. 2022;10:888827. doi: 10.3389/fbioe.2022.888827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szabo K., Teleky B.-E., Ranga F., Roman I., Khaoula H., Boudaya E., Ltaief A.B., Aouani W., Thiamrat M., Vodnar D.C. Carotenoid Recovery from Tomato Processing By-Products through Green Chemistry. Molecules. 2022;27:3771. doi: 10.3390/molecules27123771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dulf F.V., Vodnar D.C., Dulf E.H., Pintea A. Phenolic compounds, flavonoids, lipids and antioxidant potential of apricot (Prunus armeniaca L.) pomace fermented by two filamentous fungal strains in solid state system. Chem. Cent. J. 2017;11:92. doi: 10.1186/s13065-017-0323-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antonella S., Barreca D., Giuseppina L., Ersilia B., Domenico T. Bilberry (Vaccinium myrtyllus L.) Elsevier Inc.; Amsterdam, The Netherlands: 2018. [Google Scholar]
- 37.Carvalho M., Matos M., Carnide V. Identification of cultivated and wild Vaccinium species grown in Portugal. Spanish J. Agric. Res. 2018;16:e07SC01-01. doi: 10.5424/sjar/2018163-12502. [DOI] [Google Scholar]
- 38.Vaneková Z., Rollinger J.M. Bilberries: Curative and Miraculous—A Review on Bioactive Constituents and Clinical Research. Front. Pharmacol. 2022;13:2343. doi: 10.3389/fphar.2022.909914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanoeva J.P., Stefova M., Andonovska K.B., Vankova A., Stafilov T. Phenolics and mineral content in bilberry and bog bilberry from Macedonia. Int. J. Food Prop. 2017;20:S863–S883. doi: 10.1080/10942912.2017.1315592. [DOI] [Google Scholar]
- 40.Klavins L., Maaga I., Bertins M., Hykkerud A.L., Karppinen K., Bobinas Č., Salo H.M., Nguyen N., Salminen H., Stankevica K., et al. Trace element concentration and stable isotope ratio analysis in blueberries and bilberries: A tool for quality and authenticity control. Foods. 2021;10:567. doi: 10.3390/foods10030567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hera O., Teodorescu R., Sturzeanu M. Blueberry(Vaccinium Corymbosum) Breeding Programme in the Main Cultivating Countries. Sci. Pap. Ser. B, Hortic. 2021;LXV:82–89. [Google Scholar]
- 42.Martin R.R., Tzanetakis I.E. High risk blueberry viruses by region in north america; implications for certification, nurseries, and fruit production. Viruses. 2018;10:342. doi: 10.3390/v10070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rojas-Flores S., Benites S.M., De La Cruz-Noriega M., Cabanillas-Chirinos L., Valdiviezo-Dominguez F., Álvarez M.A.Q., Vega-Ybañez V., Angelats-Silva L. Bioelectricity production from blueberry waste. Processes. 2021;9:1301. doi: 10.3390/pr9081301. [DOI] [Google Scholar]
- 44.Pires T.C.S.P., Caleja C., Santos-Buelga C., Barros L., Ferreira I.C.F.R. Vaccinium myrtillus L. Fruits as a Novel Source of Phenolic Compounds with Health Benefits and Industrial Applications—A Review. Curr. Pharm. Des. 2020;26:1917–1928. doi: 10.2174/1381612826666200317132507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michalska A., Łysiak G. Bioactive compounds of blueberries: Post-harvest factors influencing the nutritional value of products. Int. J. Mol. Sci. 2015;16:18642–18663. doi: 10.3390/ijms160818642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akhlaghi M., Bandy B. Protection by Plant Flavonoids Against Myocardial Ischemia-Reperfusion Injury. Elsevier Inc.; Amsterdam, The Netherlands: 2012. [Google Scholar]
- 47.Urbonaviciene D., Bobinaite R., Viskelis P., Bobinas C., Petruskevicius A., Klavins L., Viskelis J. Geographic Variability of Biologically Active Compounds, Antioxidant Activity and Physico-Chemical Properties in Wild Bilberries (Vaccinium myrtillus L.) Antioxidants. 2022;11:588. doi: 10.3390/antiox11030588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaspar D.P., Lechtenberg M., Hensel A. Quality assessment of bilberry fruits (Vaccinium myrtillus) and bilberry-containing dietary supplements. J. Agric. Food Chem. 2021;69:2213–2225. doi: 10.1021/acs.jafc.0c07784. [DOI] [PubMed] [Google Scholar]
- 49.Gizzi C., Belcaro G., Gizzi G., Feragalli B., Dugall M., Luzzi R., Cornelli U. Bilberry extracts are not created equal: The role of non anthocyanin fraction. Discovering the “dark side of the force” in a preliminary study. Eur. Rev. Med. Pharmacol. Sci. 2016;20:2418–2424. [PubMed] [Google Scholar]
- 50.Papanov S.I., Petkova E.G., Ivanov I.G. Polyphenols Content and Antioxidant Activity of Bilberry Juice Obtained from Different Altitude Samples. J. Pharm. Res. Int. 2021:218–223. doi: 10.9734/jpri/2021/v33i29A31581. [DOI] [Google Scholar]
- 51.Ștefănescu B.E., Nemes S.A., Teleky B.E., Călinoiu L.F., Mitrea L., Martău G.A., Szabo K., Mihai M., Vodnar D.C., Crișan G. Microencapsulation and Bioaccessibility of Phenolic Compounds of Vaccinium Leaf Extracts. Antioxidants. 2022;11:674. doi: 10.3390/antiox11040674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Călinoiu L.F., Vodnar D.C. Whole grains and phenolic acids: A review on bioactivity, functionality, health benefits and bioavailability. Nutrients. 2018;10:1615. doi: 10.3390/nu10111615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heinonen M. Antioxidant activity and antimicrobial effect of berry phenolics—A Finnish perspective. Mol. Nutr. Food Res. 2007;51:684–691. doi: 10.1002/mnfr.200700006. [DOI] [PubMed] [Google Scholar]
- 54.Puupponen-Pimiä R., Nohynek L., Alakomi H.L., Oksman-Caldentey K.M. Bioactive berry compounds—Novel tools against human pathogens. Appl. Microbiol. Biotechnol. 2005;67:8–18. doi: 10.1007/s00253-004-1817-x. [DOI] [PubMed] [Google Scholar]
- 55.Davidson E., Zimmermann B.F., Jungfer E., Chrubasik-Hausmann S. Prevention of urinary tract infections with vaccinium products. Phyther. Res. 2014;28:465–470. doi: 10.1002/ptr.5047. [DOI] [PubMed] [Google Scholar]
- 56.Toivanen M., Huttunen S., Lapinjoki S., Tikkanen-Kaukanen C. Inhibition of adhesion of Neisseria meningitidis to human epithelial cells by berry juice polyphenolic fractions. Phyther. Res. 2011;25:828–832. doi: 10.1002/ptr.3349. [DOI] [PubMed] [Google Scholar]
- 57.Huttunen S., Toivanen M., Arkko S., Ruponen M., Tikkanen-Kaukanen C. Inhibition activity of wild berry juice fractions against streptococcus pneumoniae binding to human bronchial cells. Phyther. Res. 2011;25:122–127. doi: 10.1002/ptr.3240. [DOI] [PubMed] [Google Scholar]
- 58.Klavins L., Mezulis M., Nikolajeva V., Klavins M. Composition, sun protective and antimicrobial activity of lipophilic bilberry (Vaccinium myrtillus L.) and lingonberry (Vaccinium vitis-idaea L.) extract fractions. Lwt. 2021;138:110784. doi: 10.1016/j.lwt.2020.110784. [DOI] [Google Scholar]
- 59.Colak N., Torun H., Gruz J., Strnad M., Hermosín-Gutiérrez I., Hayirlioglu-Ayaz S., Ayaz F.A. Bog Bilberry Phenolics, Antioxidant Capacity and Nutrient Profile. Volume 201. Elsevier Ltd.; Amsterdam, The Netherlands: 2016. [DOI] [PubMed] [Google Scholar]
- 60.de Mello V.D.F., Lankinen M.A., Lindström J., Puupponen-Pimiä R., Laaksonen D.E., Pihlajamäki J., Lehtonen M., Uusitupa M., Tuomilehto J., Kolehmainen M., et al. Fasting serum hippuric acid is elevated after bilberry (Vaccinium myrtillus) consumption and associates with improvement of fasting glucose levels and insulin secretion in persons at high risk of developing type 2 diabetes. Mol. Nutr. Food Res. 2017;61:1700019. doi: 10.1002/mnfr.201700019. [DOI] [PubMed] [Google Scholar]
- 61.Karcheva-Bahchevanska D.P., Lukova P.K., Nikolova M.M., Mladenov R.D., Iliev I.N. Eff ect of Extracts of Bilberries (Vaccinium myrtillus L.) on Amyloglucosidase and α-Glucosidase Activity. Folia Med. (Plovdiv). 2017;59:197–202. doi: 10.1515/folmed-2017-0028. [DOI] [PubMed] [Google Scholar]
- 62.Schink A., Neumann J., Leifke A.L., Ziegler K., Fröhlich-Nowoisky J., Cremer C., Thines E., Weber B., Pöschl U., Schuppan D., et al. Screening of herbal extracts for TLR2-and TLR4-dependent anti-inflammatory effects. PLoS ONE. 2018;13:e0203907. doi: 10.1371/journal.pone.0203907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luo H., Lv X.D., Wang G.E., Li Y.F., Kurihara H., He R.R. Anti-inflammatory effects of anthocyanins-rich extract from bilberry (Vaccinium myrtillus L.) on croton oil-induced ear edema and Propionibacterium acnes plus LPS-induced liver damage in mice. Int. J. Food Sci. Nutr. 2014;65:594–601. doi: 10.3109/09637486.2014.886184. [DOI] [PubMed] [Google Scholar]
- 64.Chehri A., Yarani R., Yousefi Z., Shakouri S.K., Ostadrahimi A., Mobasseri M., Araj-Khodaei M. Phytochemical and pharmacological anti-diabetic properties of bilberries (Vaccinium myrtillus), recommendations for future studies. Prim. Care Diabetes. 2022;16:27–33. doi: 10.1016/j.pcd.2021.12.017. [DOI] [PubMed] [Google Scholar]
- 65.Bljajić K., Petlevski R., Vujić L., Čačić A., Šoštarić N., Jablan J., De Carvalho I.S., Končić M.Z. Chemical composition, antioxidant and α-glucosidase-inhibiting activities of the aqueous and hydroethanolic extracts of Vaccinium myrtillus leaves. Molecules. 2017;22:703. doi: 10.3390/molecules22050703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ungurianu A., Şeremet O., Gagniuc E., Olaru O.T., Guţu C., Grǎdinaru D., Ionescu-Tȋrgovişte C., Marginǎ D., Dǎnciulescu-Miulescu R. Preclinical and clinical results regarding the effects of a plant-based antidiabetic formulation versus well established antidiabetic molecules. Pharmacol. Res. 2019;150:104522. doi: 10.1016/j.phrs.2019.104522. [DOI] [PubMed] [Google Scholar]
- 67.Roth S., Spalinger M.R., Müller I., Lang S., Rogler G., Scharl M. Bilberry-derived anthocyanins prevent IFN-γ-induced pro-inflammatory signalling and cytokine secretion in human THP-1 monocytic cells. Digestion. 2014;90:179–189. doi: 10.1159/000366055. [DOI] [PubMed] [Google Scholar]
- 68.Ozdemir A., Mercantepe T., Erdivanli B., Sen A., Mercantepe F., Tumkaya L., Uydu H.A. Neuroprotective effects of Vaccinium myrtillus on damage-related brain injury. J. Chem. Neuroanat. 2023;127:102193. doi: 10.1016/j.jchemneu.2022.102193. [DOI] [PubMed] [Google Scholar]
- 69.Miyake S., Takahashi N., Sasaki M., Kobayashi S., Tsubota K., Ozawa Y. Vision preservation during retinal inflammation by anthocyanin-rich bilberry extract: Cellular and molecular mechanism. Lab. Investig. 2012;92:102–109. doi: 10.1038/labinvest.2011.132. [DOI] [PubMed] [Google Scholar]
- 70.Biedermann L., Mwinyi J., Scharl M., Frei P., Zeitz J., Kullak-Ublick G.A., Vavricka S.R., Fried M., Weber A., Humpf H.U., et al. Bilberry ingestion improves disease activity in mild to moderate ulcerative colitis—An open pilot study. J. Crohn’s Colitis. 2013;7:271–279. doi: 10.1016/j.crohns.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 71.Mykkänen O.T., Huotari A., Herzig K.H., Dunlop T.W., Mykkänen H., Kirjavainen P.V. Wild blueberries (Vaccinium myrtillus) alleviate inflammation and hypertension associated with developing obesity in mice fed with a high-fat diet. PLoS ONE. 2014;9:e114790. doi: 10.1371/journal.pone.0114790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim J., Kim C.S., Lee Y.M., Sohn E., Jo K., Kim J.S. Vaccinium myrtillus extract prevents or delays the onset of diabetes—Induced blood-retinal barrier breakdown. Int. J. Food Sci. Nutr. 2015;66:236–242. doi: 10.3109/09637486.2014.979319. [DOI] [PubMed] [Google Scholar]
- 73.Del Bubba M., Di Serio C., Renai L., Scordo C.V.A., Checchini L., Ungar A., Tarantini F., Bartoletti R. Vaccinium myrtillus L. extract and its native polyphenol-recombined mixture have anti-proliferative and pro-apoptotic effects on human prostate cancer cell lines. Phyther. Res. 2021;35:1089–1098. doi: 10.1002/ptr.6879. [DOI] [PubMed] [Google Scholar]
- 74.Persson I.A.L., Persson K., Andersson R.G.G. Effect of Vaccinium myrtillus and its polyphenols on angiotensin-converting enzyme activity in human endothelial cells. J. Agric. Food Chem. 2009;57:4626–4629. doi: 10.1021/jf900128s. [DOI] [PubMed] [Google Scholar]
- 75.Chen J., Uto T., Tanigawa S., Kumamoto T., Fujii M., Hou D.X. Expression profiling of genes targeted by bilberry (Vaccinium myrtillus) in macrophages through DNA microarray. Nutr. Cancer. 2008;60:43–50. doi: 10.1080/01635580802381279. [DOI] [PubMed] [Google Scholar]
- 76.Kolehmainen M., Mykkänen O., Kirjavainen P.V., Leppänen T., Moilanen E., Adriaens M., Laaksonen D.E., Hallikainen M., Puupponen-Pimiä R., Pulkkinen L., et al. Bilberries reduce low-grade inflammation in individuals with features of metabolic syndrome. Mol. Nutr. Food Res. 2012;56:1501–1510. doi: 10.1002/mnfr.201200195. [DOI] [PubMed] [Google Scholar]
- 77.Triebel S., Trieu H.L., Richling E. Modulation of inflammatory gene expression by a bilberry (Vaccinium myrtillus L.) extract and single anthocyanins considering their limited stability under cell culture conditions. J. Agric. Food Chem. 2012;60:8902–8910. doi: 10.1021/jf3028842. [DOI] [PubMed] [Google Scholar]
- 78.Simon E., Călinoiu L.F., Mitrea L., Vodnar D.C. Probiotics, prebiotics, and synbiotics: Implications and beneficial effects against irritable bowel syndrome. Nutrients. 2021;13:2112. doi: 10.3390/nu13062112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tumbas Šaponjac V., Čanadanović-Brunet J., Ćetković G., Djilas S., Četojević-Simin D. Dried bilberry (Vaccinium myrtillus L.) extract fractions as antioxidants and cancer cell growth inhibitors. Lwt. 2015;61:615–621. doi: 10.1016/j.lwt.2014.04.021. [DOI] [Google Scholar]
- 80.Mauramo M., Onali T., Wahbi W., Vasara J., Lampinen A., Mauramo E., Kivimäki A., Martens S., Häggman H., Sutinen M., et al. Bilberry (Vaccinium myrtillus L.) powder has anticarcinogenic effects on oral carcinoma in vitro and in vivo. Antioxidants. 2021;10:1319. doi: 10.3390/antiox10081319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hara S., Morita R., Ogawa T., Segawa R., Takimoto N., Suzuki K., Hamadate N., Hayashi S., Odachi A., Ogiwara I., et al. Tumor suppression effects of bilberry extracts and enzymatically modified isoquercitrin in early preneoplastic liver cell lesions induced by piperonyl butoxide promotion in a two-stage rat hepatocarcinogenesis model. Exp. Toxicol. Pathol. 2014;66:225–234. doi: 10.1016/j.etp.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 82.Nishida N., Yano H., Nishida T., Kamura T., Kojiro M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006;2:213–219. doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aiello L.P., Avery R.L., Arrigg P.G., Keyt B.A., Jampel H.D., Shah S.T., Pasquale L.R., Thieme H., Iwamoto M.A., Park J.E., et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 84.Matsunaga N., Chikaraishi Y., Shimazawa M., Yokota S., Hara H. Vaccinium myrtillus (bilberry) extracts reduce angiogenesis in vitro and in vivo. Evid.-Based Complement. Altern. Med. 2010;7:47–56. doi: 10.1093/ecam/nem151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sidorova Y., Shipelin V., Mazo V., Zorin S., Petrov N., Kochetkova A. Hypoglycemic and hypolipidemic effect of Vaccinium myrtillus L. leaf and Phaseolus vulgaris L. seed coat extracts in diabetic rats. Nutrition. 2017;41:107–112. doi: 10.1016/j.nut.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 86.Varut R.M., Gîrd C.E., Rotaru L.T., Varut M.C., Pisoschi C.G. Evaluation of Polyphenol and Flavonoid Profiles and the Antioxidant Effect of Carduus Acanthoides Hydroalcoholic Extract Compared with Vaccinium myrtillus in an Animal Model of Diabetes Mellitus. Pharm. Chem. J. 2018;51:1088–1095. doi: 10.1007/s11094-018-1746-0. [DOI] [Google Scholar]
- 87.Grace M.H., Ribnicky D.M., Kuhn P., Poulev A., Logendra S., Yousef G.G., Raskin I., Lila M.A. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine. 2009;16:406–415. doi: 10.1016/j.phymed.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Asgary S., Rafieiankopaei M., Sahebkar A., Shamsi F., Goli-malekabadi N. Anti-hyperglycemic and anti-hyperlipidemic effects of Vaccinium myrtillus fruit in experimentally induced diabetes (antidiabetic effect of Vaccinium myrtillus fruit) J. Sci. Food Agric. 2016;96:764–768. doi: 10.1002/jsfa.7144. [DOI] [PubMed] [Google Scholar]
- 89.Chen K., Wei X., Zhang J., Pariyani R., Jokioja J., Kortesniemi M., Linderborg K.M., Heinonen J., Sainio T., Zhang Y., et al. Effects of anthocyanin extracts from bilberry (Vaccinium myrtillus L.) and purple potato (Solanum tuberosum L. var. ’Synkeä Sakari’) on the plasma metabolomic profile of Zucker diabetic fatty rats. J. Agric. Food Chem. 2020;68:9436–9450. doi: 10.1021/acs.jafc.0c04125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kowalska K., Olejnik A., Szwajgier D., Olkowicz M. Inhibitory activity of chokeberry, bilberry, raspberry and cranberry polyphenol-rich extract towards adipogenesis and oxidative stress in differentiated 3T3-L1 adipose cells. PLoS ONE. 2017;12:e0188583. doi: 10.1371/journal.pone.0188583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kowalska K., Olejnik A., Rychlik J., Grajek W. Cranberries (Oxycoccus quadripetalus) inhibit lipid metabolism and modulate leptin and adiponectin secretion in 3T3-L1 adipocytes. Food Chem. 2015;185:383–388. doi: 10.1016/j.foodchem.2015.03.152. [DOI] [PubMed] [Google Scholar]
- 92.Suzuki R., Tanaka M., Takanashi M., Hussain A., Yuan B., Toyoda H., Kuroda M. Anthocyanidins-enriched bilberry extracts inhibit 3T3-L1 adipocyte differentiation via the insulin pathway. Nutr. Metab. 2011;8:14. doi: 10.1186/1743-7075-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brader L., Overgaard A., Christensen L.P., Jeppesen P.B., Hermansen K. Polyphenol-rich bilberry ameliorates total cholesterol and LDL-cholesterol when implemented in the diet of Zucker diabetic fatty rats. Rev. Diabet. Stud. 2013;10:270–282. doi: 10.1900/RDS.2013.10.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frostegård J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013;11:117. doi: 10.1186/1741-7015-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mauray A., Felgines C., Morand C., Mazur A., Scalbert A., Milenkovic D. Nutrigenomic analysis of the protective effects of bilberry anthocyanin-rich extract in apo E-deficient mice. Genes Nutr. 2010;5:343–353. doi: 10.1007/s12263-010-0171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mauray A., Felgines C., Morand C., Mazur A., Scalbert A., Milenkovic D. Bilberry anthocyanin-rich extract alters expression of genes related to atherosclerosis development in aorta of apo E-deficient mice. Nutr. Metab. Cardiovasc. Dis. 2012;22:72–80. doi: 10.1016/j.numecd.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 97.Ferlemi A.V., Lamari F.N. Berry leaves: An alternative source of bioactive natural products of nutritional and medicinal value. Antioxidants. 2016;5:17. doi: 10.3390/antiox5020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Erlund I., Koli R., Alfthan G., Marniemi J., Puukka P., Mustonen P., Mattila P., Jula A. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am. J. Clin. Nutr. 2008;87:323–331. doi: 10.1093/ajcn/87.2.323. [DOI] [PubMed] [Google Scholar]
- 99.Lankinen M., Kolehmainen M., Jääskeläinen T., Paananen J., Joukamo L., Kangas A.J., Soininen P., Poutanen K., Mykkänen H., Gylling H., et al. Effects of whole grain, fish and bilberries on serum metabolic profile and lipid transfer protein activities: A randomized trial (Sysdimet) PLoS ONE. 2014;9:e90352. doi: 10.1371/journal.pone.0090352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Diaz-Garcia L., Schlautman B., Covarrubias-Pazaran G., Maule A., Johnson-Cicalese J., Grygleski E., Vorsa N., Zalapa J. Massive phenotyping of multiple cranberry populations reveals novel QTLs for fruit anthocyanin content and other important chemical traits. Mol. Genet. Genom. 2018;293:1379–1392. doi: 10.1007/s00438-018-1464-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bolivar-Medina J.L., Villouta C., Workmaster B.A., Atucha A. Floral meristem development in cranberry apical buds during winter rest and its implication on yield prediction. J. Am. Soc. Hortic. Sci. 2019;144:314–320. doi: 10.21273/JASHS04691-19. [DOI] [Google Scholar]
- 102.Feghali K., Feldman M., La V.D., Santos J., Grenier D. Cranberry proanthocyanidins: Natural weapons against periodontal diseases. J. Agric. Food Chem. 2012;60:5728–5735. doi: 10.1021/jf203304v. [DOI] [PubMed] [Google Scholar]
- 103.Diaz-Garcia L., Rodriguez-Bonilla L., Phillips M., Lopez-Hernandez A., Grygleski E., Atucha A., Zalapa J. Comprehensive analysis of the internal structure and firmness in American cranberry (Vaccinium macrocarpon Ait.) fruit. PLoS ONE. 2019;14:e0222451. doi: 10.1371/journal.pone.0222451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rodriguez-Bonilla L., Williams K.A., Rodríguez Bonilla F., Matusinec D., Maule A., Coe K., Wiesman E., Diaz-Garcia L., Zalapa J. The genetic diversity of cranberry crop wild relatives, Vaccinium macrocarpon aiton and v. Oxycoccos l., in the us, with special emphasis on national forests. Plants. 2020;9:1446. doi: 10.3390/plants9111446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sandler H., DeMoranville C. Cranberry Production: A Guide for Massachusetts—Summary Edition. UMass Amherst Cranberry Stn. 2008:1–37. [Google Scholar]
- 106.Neto C.C. Cranberry and its phytochemicals: A review of in vitro anticancer studies. J. Nutr. 2007;137:186S–193S. doi: 10.1093/jn/137.1.186S. [DOI] [PubMed] [Google Scholar]
- 107.Karlsons A., Osvalde A., Nollendorfs V. Research on the mineral composition of american cranberries and wild cranberries in Latvia. Latv. J. Agron. 2009;12:65–71. [Google Scholar]
- 108.Česonienė L., Daubaras R. Phytochemical Composition of the Large Cranberry (Vaccinium macrocarpon) and the Small Cranberry (Vaccinium oxycoccos) Elsevier Inc.; Amsterdam, The Netherlands: 2015. [Google Scholar]
- 109.Martinussen I., Rohloff J., Uleberg E., Junttila O., Hohtola A., Jaakola L., Häggman H. Climatic effects on the production and quality of bilberries (Vaccinium myrtillus) Agron. Vēstis. 2009:71–74. [Google Scholar]
- 110.Kalın P., Gülçin İl., Gören A.C. Antioxidant activity and polyphenol content of cranberries (Vaccinium macrocarpon) Rec. Nat. Prod. 2015;9:496–502. [Google Scholar]
- 111.Nemzer B.V., Al-Taher F., Yashin A., Revelsky I., Yashin Y. Cranberry: Chemical Composition, Antioxidant Activity and Impact on Human Health. Overview. Molecules. 2022;27:1503. doi: 10.3390/molecules27051503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Oszmiański J., Lachowicz S., Gorzelany J., Matłok N. The effect of different maturity stages on phytochemical composition and antioxidant capacity of cranberry cultivars. Eur. Food Res. Technol. 2018;244:705–719. doi: 10.1007/s00217-017-2994-z. [DOI] [Google Scholar]
- 113.Rózańska D., Regulska-Ilow B. The significance of anthocyanins in the prevention and treatment of type 2 diabetes. Adv. Clin. Exp. Med. 2018;27:135–142. doi: 10.17219/acem/64983. [DOI] [PubMed] [Google Scholar]
- 114.Babar A., Moore L., Leblanc V., Dudonné S., Desjardins Y., Lemieux S., Bochard V., Guyonnet D., Dodin S. Standardised high dose versus low dose cranberry Proanthocyanidin extracts for the prevention of recurrent urinary tract infection in healthy women [PACCANN]: A double blind randomised controlled trial protocol. BMC Urol. 2021;21:29. doi: 10.1186/s12894-021-00811-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Blumberg J.B., Camesano T.A., Cassidy A., Kris-Etherton P., Howell A., Manach C., Ostertag L.M., Sies H., Skulas-Ray A., Vita J.A. Cranberries and their bioactive constituents in human health. Adv. Nutr. 2013;4:618–632. doi: 10.3945/an.113.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Krueger C.G., Reed J.D., Feliciano R.P., Howell A.B. Quantifying and characterizing proanthocyanidins in cranberries in relation to urinary tract health. Anal. Bioanal. Chem. 2013;405:4385–4395. doi: 10.1007/s00216-013-6750-3. [DOI] [PubMed] [Google Scholar]
- 117.Oszmiański J., Kolniak-Ostek J., Lachowicz S., Gorzelany J., Matłok N. Phytochemical Compounds and Antioxidant Activity in Different Cultivars of Cranberry (Vaccinium macrocarpon L) J. Food Sci. 2017;82:2569–2575. doi: 10.1111/1750-3841.13924. [DOI] [PubMed] [Google Scholar]
- 118.Wu X., Wu X., Xue L., Tata A., Song M., Song M., Neto C.C., Xiao H. Bioactive Components of Polyphenol-Rich and Non-Polyphenol-Rich Cranberry Fruit Extracts and Their Chemopreventive Effects on Colitis-Associated Colon Cancer. J. Agric. Food Chem. 2020;68:6845–6853. doi: 10.1021/acs.jafc.0c02604. [DOI] [PubMed] [Google Scholar]
- 119.Narwojsz A., Tańska M., Mazur B., Borowska E.J. Fruit Physical Features, Phenolic Compounds Profile and Inhibition Activities of Cranberry Cultivars (Vaccinium macrocarpon) Compared to Wild-Grown Cranberry (Vaccinium oxycoccus) Plant Foods Hum. Nutr. 2019;74:300–306. doi: 10.1007/s11130-019-00737-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Noushahi H.A., Khan A.H., Noushahi U.F., Hussain M., Javed T., Zafar M., Batool M., Ahmed U., Liu K., Harrison M.T., et al. Biosynthetic pathways of triterpenoids and strategies to improve their Biosynthetic Efficiency. Plant Growth Regul. 2022;97:439–454. doi: 10.1007/s10725-022-00818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sedbare R., Raudone L., Zvikas V., Viskelis J., Liaudanskas M., Janulis V. Development and Validation of the UPLC-DAD Methodology for the Detection of Triterpenoids and Phytosterols in Fruit Samples of Vaccinium macrocarpon Aiton and Vaccinium oxycoccos L. Molecules. 2022;27:4403. doi: 10.3390/molecules27144403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Viskelis P., Rubinskiene M., Jasutiene I., Šarkinas A., Daubaras R., Česoniene L. Anthocyanins, antioxidative, and antimicrobial properties of american cranberry (Vaccinium macrocarpon ait.) and their press cakes. J. Food Sci. 2009;74:C157–C161. doi: 10.1111/j.1750-3841.2009.01066.x. [DOI] [PubMed] [Google Scholar]
- 123.Daoutidou M., Plessas S., Alexopoulos A. Assessment of Antimicrobial Activity of Pomegranate, Cranberry, and Black Chokeberry Extracts against Foodborne Pathogens. Foods. 2021;10:486. doi: 10.3390/foods10030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kranz S., Guellmar A., Olschowsky P., Tonndorf-Martini S., Heyder M., Pfister W., Reise M., Sigusch B. Antimicrobial Effect of Natural Berry Juices on Common Oral Pathogenic Bacteria. Antibiotics. 2019;9:533. doi: 10.3390/antibiotics9090533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Howell A.B. Potential of cranberry for suppressing Helicobacter pylori, a risk factor for gastric cancer. J. Berry Res. 2020;10:11–20. doi: 10.3233/JBR-180375. [DOI] [Google Scholar]
- 126.Burger O., Ofek I., Tabak M., Weiss E.I., Sharon N., Neeman I. A high molecular mass constituent of cranberry juice inhibits Helicobacter pylori adhesion to human gastric mucus. FEMS Immunol. Med. Microbiol. 2000;29:295–301. doi: 10.1111/j.1574-695X.2000.tb01537.x. [DOI] [PubMed] [Google Scholar]
- 127.Kowalska K., Olejnik A. Beneficial effects of cranberry in the prevention of obesity and related complications: Metabolic syndrome and diabetes—A review. J. Funct. Foods. 2016;20:171–181. doi: 10.1016/j.jff.2015.11.001. [DOI] [Google Scholar]
- 128.Anhê F.F., Roy D., Pilon G., Dudonné S., Matamoros S., Varin T.V., Garofalo C., Moine Q., Desjardins Y., Levy E., et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2015;64:872–883. doi: 10.1136/gutjnl-2014-307142. [DOI] [PubMed] [Google Scholar]
- 129.Kunkel S.D., Elmore C.J., Bongers K.S., Ebert S.M., Fox D.K., Dyle M.C., Bullard S.A., Adams C.M. Ursolic acid increases skeletal muscle and brown fat and decreases diet-induced obesity, glucose intolerance and fatty liver disease. PLoS ONE. 2012;7:e39332. doi: 10.1371/journal.pone.0039332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cermak R., Landgraf S., Wolffram S. Quercetin glucosides inhibit glucose uptake into brush-border-membrane vesicles of porcine jejunum. Br. J. Nutr. 2004;91:849–855. doi: 10.1079/BJN20041128. [DOI] [PubMed] [Google Scholar]
- 131.Schell J., Betts N.M., Foster M., Scofield R.H., Basu A. Cranberries improve postprandial glucose excursions in type 2 diabetes. Food Funct. 2017;8:3083–3090. doi: 10.1039/C7FO00900C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.de Llano D.G., Moreno-Arribas M.V., Bartolomé B. Cranberry polyphenols and prevention against urinary tract Infections: Relevant considerations. Molecules. 2020;25:3523. doi: 10.3390/molecules25153523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alexander B. Oral Health Benefits of Cranberry: A Review. IOSR J. Dent. Med. Sci. 2019;18:41–44. doi: 10.9790/0853-1801024144. [DOI] [Google Scholar]
- 134.Nowaczyk P.M., Bajerska J., Lasik-Kurdyś M., Radziejewska-Kubzdela E., Szwengiel A., Woźniewicz M. The effect of cranberry juice and a cranberry functional beverage on the growth and metabolic activity of selected oral bacteria. BMC Oral Health. 2021;21:660. doi: 10.1186/s12903-021-02025-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ruel G., Couillard C. Evidences of the cardioprotective potential of fruits: The case of cranberries. Mol. Nutr. Food Res. 2007;51:692–701. doi: 10.1002/mnfr.200600286. [DOI] [PubMed] [Google Scholar]
- 136.Masnadi Shirazi K., Shirinpour E., Masnadi Shirazi A., Nikniaz Z. Effect of cranberry supplementation on liver enzymes and cardiometabolic risk factors in patients with NAFLD: A randomized clinical trial. BMC Complement. Med. Ther. 2021;21:283. doi: 10.1186/s12906-021-03436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thimóteo N.S.B., Iryioda T.M.V., Alfieri D.F., Rego B.E.F., Scavuzzi B.M., Fatel E., Lozovoy M.A.B., Simão A.N.C., Dichi I. Cranberry juice decreases disease activity in women with rheumatoid arthritis. Nutrition. 2019;60:112–117. doi: 10.1016/j.nut.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 138.McKay D.L., Blumberg J.B. Cranberries (Vaccinium macrocarpon) and cardiovascular disease risk factors. Nutr. Rev. 2007;65:490–502. doi: 10.1301/nr.2007.nov.490-502. [DOI] [PubMed] [Google Scholar]
- 139.Mantzorou M., Zarros A., Vasios G., Theocharis S., Pavlidou E., Giaginis C. Cranberry: A Promising Natural Source of Potential Nutraceuticals with Anticancer Activity. Anticancer. Agents Med. Chem. 2019;19:1672–1686. doi: 10.2174/1871520619666190704163301. [DOI] [PubMed] [Google Scholar]
- 140.Strobel P., Allard C., Perez-Acle T., Calderon R., Aldunate R., Leighton F. Myricetin, quercetin and catechin-gallate inhibit glucose uptake in isolated rat adipocytes. Biochem. J. 2005;386:471–478. doi: 10.1042/BJ20040703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rocha D.M.U.P., Caldas A.P.S., da Silva B.P., Hermsdorff H.H.M., Alfenas R. de C.G. Effects of blueberry and cranberry consumption on type 2 diabetes glycemic control: A systematic review. Crit. Rev. Food Sci. Nutr. 2019;59:1816–1828. doi: 10.1080/10408398.2018.1430019. [DOI] [PubMed] [Google Scholar]
- 142.Debnath S.C., Barney D.L. Shoot regeneration and plantlet formation by cascade huckleberry, mountain huckleberry, and oval-leaf bilberry on a Zeatin-containing nutrient medium. Horttechnology. 2012;22:106–113. doi: 10.21273/HORTTECH.22.1.106. [DOI] [Google Scholar]
- 143.Hummer K.E. Manna in winter: Indigenous Americans, huckleberries, and blueberries. HortScience. 2013;48:413–417. doi: 10.21273/HORTSCI.48.4.413. [DOI] [Google Scholar]
- 144.Lila M.A., Kellogg J., Grace M.H., Yousef G.G., Kraft T.B., Rogers R.B. Stressed for success: How the berry’s wild origins result in multifaceted health protections. Acta Hortic. 2014;1017:23–44. doi: 10.17660/ActaHortic.2014.1017.1. [DOI] [Google Scholar]
- 145.Lee J., Finn C.E., Wrolstad R.E. Anthocyanin pigment and total phenolic content of three Vaccinium species native to the Pacific Northwest of North America. HortScience. 2004;39:959–964. doi: 10.21273/HORTSCI.39.5.959. [DOI] [PubMed] [Google Scholar]
- 146.Kerns B.K., Alexander S.J., Bailey J.D. Huckleberry abundance, stand conditions, and use in Western Oregon: Evaluating the role of forest management. Econ. Bot. 2004;58:668–678. doi: 10.1663/0013-0001(2004)058[0668:HASCAU]2.0.CO;2. [DOI] [Google Scholar]
- 147.Finn C., Young A. Variability for Reproductive and Vegetative Traits in Vaccinium Membranaceum. Acta Hortic. 2002:181–187. doi: 10.17660/ActaHortic.2002.574.27. [DOI] [Google Scholar]
- 148.Stevens M., Darris D.C. [(accessed on 22 November 2022)];Black Huckleberry Vaccinium membranaceum Dougl. ex Torr. 2001 USDA NRCS National Plant Data Center & Oregon Plant Materials Center, United States. Available online: http://plant-materials.nrcs.usda.gov.
- 149.Taruscio T.G., Barney D.L., Exon J. Content and Profile of Flavanoid and Phenolic Acid Compounds in Conjunction with the Antioxidant Capacity for a Variety of Northwest Vaccinium Berries. J. Agric. Food Chem. 2004;52:3169–3176. doi: 10.1021/jf0307595. [DOI] [PubMed] [Google Scholar]
- 150.Vander Kloet S.P., Dickinson T.A. The taxonomy of Vaccinium section Myrtillus (Ericaceae) Brittonia. 1999;51:231–254. doi: 10.2307/2666632. [DOI] [Google Scholar]
- 151.Barney D.L. Prospects for domesticating western huckleberries. Small Fruits Rev. 2003;2:15–29. doi: 10.1300/J301v02n01_03. [DOI] [Google Scholar]
- 152.Barney D.L., Lopez O.A., King E. Micropropagation of cascade huckleberry, mountain huckleberry, and oval-leaf bilberry using woody plant medium and Murashige and Skoog medium formulations. Horttechnology. 2007;17:279–284. doi: 10.21273/HORTTECH.17.3.279. [DOI] [Google Scholar]
- 153.Gorzelak M.A., Hambleton S., Massicotte H.B. Community structure of ericoid mycorrhizas and root-associated fungi of Vaccinium membranaceum across an elevation gradient in the Canadian Rocky Mountains. Fungal Ecol. 2012;5:36–45. doi: 10.1016/j.funeco.2011.08.008. [DOI] [Google Scholar]
- 154.Holden Z.A., Kasworm W.F., Servheen C., Hahn B., Dobrowski S. Sensitivity of berry productivity to climatic variation in the cabinet-yaak grizzly bear recovery zone, Northwest United States, 1989–2010. Wildl. Soc. Bull. 2012;36:226–231. doi: 10.1002/wsb.128. [DOI] [Google Scholar]
- 155.Lee J., Finn C.E., Wrolstad R.E. Comparison of anthocyanin pigment and other phenolic compounds of Vaccinium membranaceum and Vaccinium ovatum native to the Pacific Northwest of North America. J. Agric. Food Chem. 2004;52:7039–7044. doi: 10.1021/jf049108e. [DOI] [PubMed] [Google Scholar]
- 156.Kuhnlein H.V. Nutrient values in indigenous wild plant greens and roots used by the Nuxalk people of Bella Coola, British Columbia. J. Food Compos. Anal. 1990;3:38–46. doi: 10.1016/0889-1575(90)90007-9. [DOI] [Google Scholar]
- 157.Prevéy J.S., Parker L.E., Harrington C.A., Lamb C.T., Proctor M.F. Climate change shifts in habitat suitability and phenology of huckleberry (Vaccinium membranaceum) Agric. For. Meteorol. 2020;280:107803. doi: 10.1016/j.agrformet.2019.107803. [DOI] [Google Scholar]
- 158.Shores C.R., Mikle N., Graves T.A. Mapping a keystone shrub species, huckleberry (Vaccinium membranaceum), using seasonal colour change in the Rocky Mountains. Int. J. Remote Sens. 2019;40:5695–5715. doi: 10.1080/01431161.2019.1580819. [DOI] [Google Scholar]
- 159.Nemes S.A., Szabo K. Applicability of Agro-Industrial By-Products in Intelligent Food Packaging. Coatings. 2020;10:550. doi: 10.3390/coatings10060550. [DOI] [Google Scholar]
- 160.Fărcaș A.C., Socaci S.A., Nemeș S.A., Pop O.L., Coldea T.E., Fogarasi M., Biriș-Dorhoi E.S. An Update Regarding the Bioactive Compound of Cereal By-Products: Health Benefits and Potential Applications. Nutrients. 2022;14:3470. doi: 10.3390/nu14173470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mitrea L., Călinoiu L.-F., Precup G., Bindea M., Rusu B., Trif M., Ferenczi L.-J., Ştefănescu B.-E., VODNAR D.C. Inhibitory Potential Of Lactobacillus Plantarum on Escherichia Coli. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Food Sci. Technol. 2017;74:99. doi: 10.15835/buasvmcn-fst:0031. [DOI] [Google Scholar]
- 162.Teleky B.-E., Mitrea L., Călinoiu L.-F., Martău G.-A., Vodnar D.-C. Reference Module in Food Science. Elsevier; Amsterdam, The Netherlands: 2023. Microbial Processes to Produce Food Ingredients and Products; pp. 1–17. [Google Scholar]
- 163.Booth N.L., Kruger C.L., Wallace Hayes A., Clemens R. An innovative approach to the safety evaluation of natural products: Cranberry (Vaccinium macrocarpon Aiton) leaf aqueous extract as a case study. Food Chem. Toxicol. 2012;50:3150–3165. doi: 10.1016/j.fct.2012.03.075. [DOI] [PubMed] [Google Scholar]
- 164.Stefkov G., Hristovski S., Petreska Stanoeva J., Stefova M., Melovski L., Kulevanova S. Resource assessment and economic potential of bilberries (Vaccinium myrtillus and Vaccinium uliginosum) on Osogovo Mtn., R. Macedonia. Ind. Crops Prod. 2014;61:145–150. doi: 10.1016/j.indcrop.2014.06.053. [DOI] [Google Scholar]
- 165.Holopainen M., Jahodar L., Seppanen-Laakso T., Laakso I., Kauppinen V. Antimicrobial activity of some Finnish Ericaceous plants. Acta Pharm. Fenn. 1988;97:197–202. [Google Scholar]
- 166.Riihinen K., Jaakola L., Kärenlampi S., Hohtola A. Organ-specific distribution of phenolic compounds in bilberry (Vaccinium myrtillus) and “northblue” blueberry (Vaccinium corymbosum x V. angustifolium) Food Chem. 2008;110:156–160. doi: 10.1016/j.foodchem.2008.01.057. [DOI] [PubMed] [Google Scholar]
- 167.Martz F., Jaakola L., Julkunen-Tiitto R., Stark S. Phenolic Composition and Antioxidant Capacity of Bilberry (Vaccinium myrtillus) Leaves in Northern Europe Following Foliar Development and Along Environmental Gradients. J. Chem. Ecol. 2010;36:1017–1028. doi: 10.1007/s10886-010-9836-9. [DOI] [PubMed] [Google Scholar]
- 168.Rouanet J.M., Décordé K., Del Rio D., Auger C., Borges G., Cristol J.P., Lean M.E.J., Crozier A. Berry juices, teas, antioxidants and the prevention of atherosclerosis in hamsters. Food Chem. 2010;118:266–271. doi: 10.1016/j.foodchem.2009.04.116. [DOI] [Google Scholar]
- 169.Wang S.Y., HsinShan L. Antioxidant Activity in Fruits and Leaves of Blackberry, Raspberry, and Strawberry Varies with Cultivar and Developmental Stage. J. Agric. Food Chem. 2000;48:140–146. doi: 10.1021/jf9908345. [DOI] [PubMed] [Google Scholar]
- 170.Nile S.H., Park S.W. Edible berries: Bioactive components and their effect on human health. Nutrition. 2014;30:134–144. doi: 10.1016/j.nut.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 171.Kitrytė V., Kavaliauskaitė A., Tamkutė L., Pukalskienė M., Syrpas M., Rimantas Venskutonis P. Zero waste biorefining of lingonberry (Vaccinium vitis-idaea L.) pomace into functional ingredients by consecutive high pressure and enzyme assisted extractions with green solvents. Food Chem. 2020;322:126767. doi: 10.1016/j.foodchem.2020.126767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.