Table 1.
Phenolic compounds identified in aerial parts of Vaccinium plants.
| Class | Phenolic Compounds | Chemical Structures (Main Compound) | |
|---|---|---|---|
| Leaves | Stems | ||
| Catechins | (+)-catechin—R1 = H (–)-epicatechin—R1 = H+ gallocatechin—R1 = OH epigallocatechin—R1 = OH |
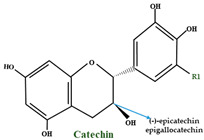
|
|
| Cinchonains | cinchonains I cinchonains II |
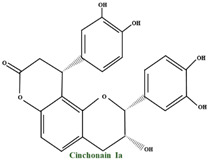
|
|
| Phenolic acids | 3,4–dihydroxybenzoic p–coumaroyl quinic acid isomers p–coumaroyl malonic acid p–coumaroyl derivatives p–coumaroyl glucose coumaroyl iridoid |
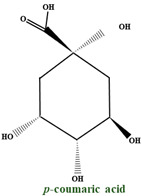
|
|
| p–coumaric acid | - |
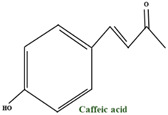
|
|
| feruloyl quinic acid isomer | - | ||
| caffeoyl quinic acid isomers | - | ||
| caffeic acid ethyl ester | - | ||
| caffeic acid hexoside | - | ||
| Proantho- cyanidins |
B–type dimer B–type trimer B–type tetramer B–type pentamer A–type dimer A–type trimer procyanidin A2 procyanidin B1 procyanidin B2 |
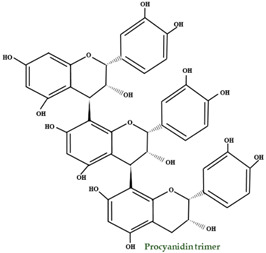
|
|
| Flavonols | quercetin–3–O–(4”–HMG)–α–rhamnoside quercetin–3–O–galactoside quercetin–3–O–glucoside quercetin–3–O–rutinoside quercetin–3–O-α–rhamnoside quercetin–3–O–arabinoside quercetin–3–O–glucuronide |
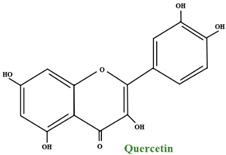
|
|
| kaempferol | - |
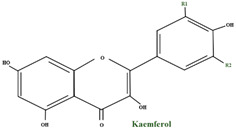
|
|
| kaempferol–3–glucuronide | - | ||
| kaempferol–hexoside | - | ||
| kaempferol–O–pentoside | - | ||
| kaempferol–(HMG)–rhamnoside | - | ||
| Lignans | - | Lyoniside (9–O–β–D–xylopyranosyl(+)lyoniresinol) |
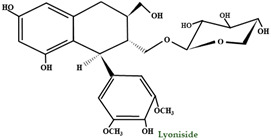
|
