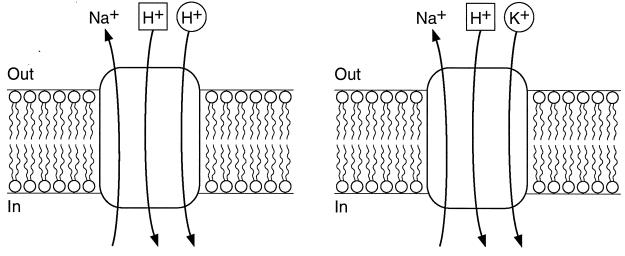
FIG. 9.
Proposed antiport activities of GerN. Two modes of GerN-mediated antiport are shown. (Left) GerN-mediated Na+/H+ antiport. The assignment of a stoichiometry of 2H+ entering in exchange for 1 Na+ effluxing is completely hypothetical and represents the apparent electrogenicity of the antiport, i.e., the number of total coupling ions translocated per turnover is greater than the number of effluxing Na+ ions, so that a net positive charge moves inward. The geometric figures surrounding the two coupling ions suggest that these ions have distinct binding sites. The pH profile of Na+/H+ antiport suggests that protons compete with Na+ on the cytoplasmic side of the membrane. (Right) Na+/H+-K+ antiport by GerN is supported by the the finding of GerN-mediated and Na+-dependent Rb+ (K+) translocation with K+ replacing some, but not all, of the H+ ions that are transported in antiport with Na+. In the diagram, K+ is shown, hypothetically, as able to compete with H+ at only one of the H+ binding sites, and the antiport is shown as still requiring the full complement of coupling ions in this mode. GerN-mediated Na+/H+-K+ antiport is much more rapid than Na+/H+ antiport; K+ increases the velocity of the antiport without affecting the Km for Na+.