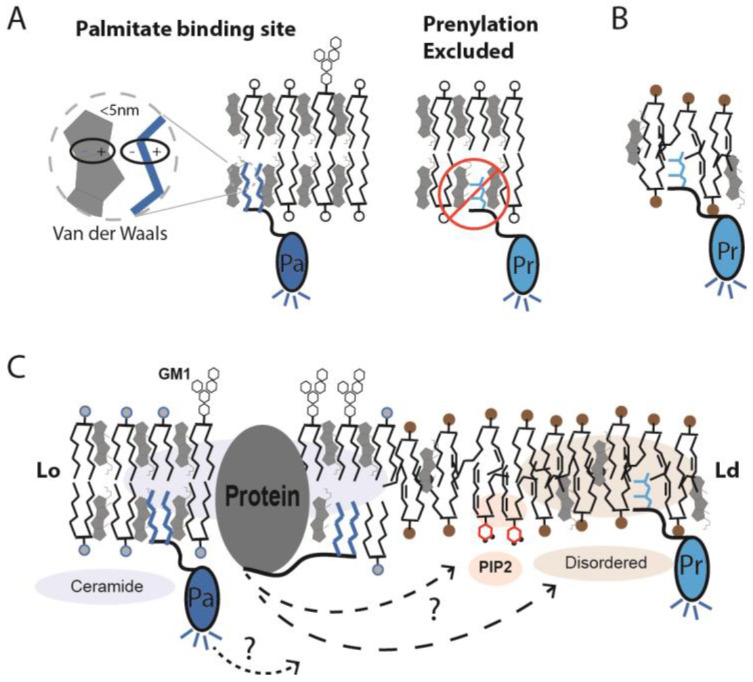
Figure 3.
Palmitate binding site in ordered lipids. (A) A model depicting the molecular basis for GM1 lipids selectively binding to palmitoylated protein (Pa). GM1 lipids are saturated and pack well with cholesterol and palmitate. The site is specific and does not bind prenylated (Pr) proteins. The branched structure of prenyl lipids is thought to clash with the structured palmitate site, thus excluding prenylated proteins from ordered lipid domains (Lo). When attached to a fluorescent protein (dark blue shaded protein), the palmitoylation becomes a sensor for the order of GM1 lipids. (B) The prenylation binding site is shown within disordered lipids (unsaturated with bent acyl chains). When coupled to a genetically encoded fluorescent protein (light blue shading), the prenylated protein functions as a sensor of prenyl localization. (C) In cellular membranes, the covalent attachment of a palmitate or a prenyl lipid to a protein tags the protein for sorting between GM1 and disordered lipids, respectively. If the protein is a fluorescent protein, the respective compartments are fluorescently labeled. (B) When the order of the GM1 lipids is decreased, the palmitate binding site is disrupted, and the palmitoylated proteins can move away from the GM1 lipids. Some proteins move to PIP2 clusters (red shading), some move to disordered lipids (tan shading), and some proteins may remain clustered with GM1. Without labeling the lipid, the movement of proteins between lipids is unclear, as depicted by a ‘?’ in the figure.