Abstract
Immunotherapy is crucial in fighting cancer and achieving successful remission. Many novel strategies have recently developed, but there are still some obstacles to overcome before we can effectively attack the cancer cells and decimate the cancer environment by inducing a cascade of immune responses. To successfully demonstrate antitumor activity, immune cells must be delivered to cancer cells and exposed to the immune system. Such cutting-edge technology necessitates meticulously designed delivery methods with no loss or superior homing onto cancer environments, as well as high therapeutic efficacy and fewer adverse events. In this paper, we discuss recent advances in cancer immunotherapy delivery techniques, as well as their future prospects.
Keywords: drug delivery, cancer immunotherapy, CAR-T cells, nanoparticles
1. Introduction
Many strategies for inducing an antitumor response against cancers have been developed. Conventional treatment modalities include surgery, radiotherapy, and chemotherapy, each of which has had advantages and disadvantages with limited success in improving clinical outcomes. A thorough understanding of how tumors interact with the host immune system will aid in development of cancer therapeutics [1]. The tumor microenvironment is a complex and dynamic network of cellular and non-cellular matrix, a complex cluster of malignant cells, tumor stromal cells, extracellular matrices, blood vessels, immune cells, and signaling molecules, which influences the response to antitumor activity. They interact within TMEs in conjunction with some signaling molecules, cytokines, and chemokines in a sophisticated manner for the growth and metastasis of cells. Tumor cells construct an immunosuppressive environment to promote tumor growth and immune evasion mechanism through mechanisms such as inhibiting the Th1 cells, which in turn induces cytotoxic T cell differentiation and Th2 antagonistic response. Designing a cancer immunotherapy with appropriate delivery modalities will overcome the drawbacks associated with cancer [2].
Cancer employs a variety of strategies to evade the immune system, including delaying or even stopping antitumor activities. These immune-evading mechanisms overpower the natural immune system’s antitumor activity, and promote tumor formation, and metastasis. These mechanisms continue to evolve as cancer progresses, becoming more diverse and complex in late-stage malignancies. Various host factors that make up the immune system influence treatment outcomes, which can lead to disease progression or regression. Blocking these immune evasion strategies had led to the discovery of new strategies for strengthening the immune response against cancer [3]. Recent advances in cancer biology and anticancer immunity, most notably the identification of numerous key immunosuppressive pathways, have greatly aided this immunotherapeutic revolution. The 2018 Nobel Prize in Physiology or Medicine was awarded to James Allison and Tasuku Honjo for their “discovery of cancer therapy through inhibition of negative immune regulation.” Specifically, the Nobel Prize was awarded for the identification of immune checkpoints (i.e., cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and programmed death/ligand 1 (PD-1/PD-L1)), which led to the development of antibodies targeting these checkpoints for anticancer treatment [4]. Cancer immunotherapy has revolutionized cancer treatment. In contrast to chemotherapies and other treatments that directly destroy cancer cells, these medications aim to boost antitumor immune responses with fewer side effects [4].
Various strategies are employed by tumors to evade the immune system, such as downregulating antigen processing or presenting machinery (MHCI, proteosome subunit latent membrane protein 2 (LMP 2) and LMP 7, transport associated with antigen processing (TAP) protein and tapasin) as to not be recognized by T cells, which is the triggering point of recognizing and attacking tumor. Another strategy employed by tumors is to downregulate IFN signaling. which may evade antigen presentation and subsequently result in evasion from the immune system. Cancer cells also cause T cell exhaustion by increasing the PD-1 and PD-L1 expression by various inflammatory and oncogenic signals leading to immune evasion. Other immune suppressive modulators such as TGF-γ, IL-8, IL-10, VEGF can be secreted into the TME by tumor cells, which in turn suppress dendritic cell maturation and T cell functions. Tumor cells may suppress T cell function by manipulating the metabolic composition in the TME to wither its activity effectively [5].
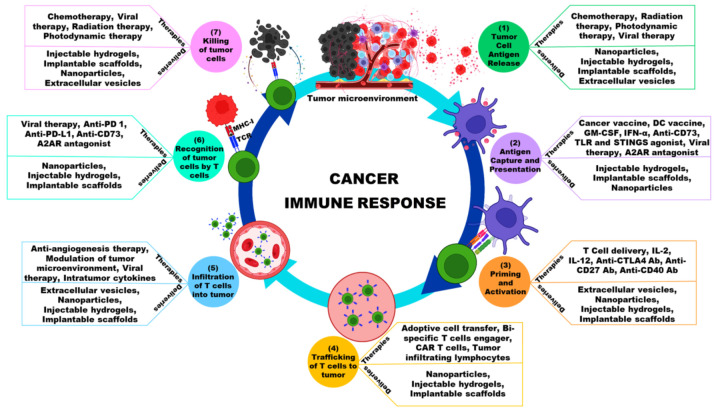
The cancer–immunity cycle is a schematic representation of the principles of cancer immunotherapy. This cycle begins with the release of tumor antigens, which are taken up, processed, and presented to naive T cells (APCs). As a result, cytotoxic T cells are produced that can specifically recognize and kill cancer cells. Lysed cancer cells then release antigens and costimulatory signals, triggering another round of the immune response cascade. Tumors can disrupt critical elements of the cancer–immunity cycle via a variety of negative feedback immune regulatory pathways, which are increasingly becoming cancer immunotherapy targets [6]. These treatments aim to boost antitumor immune responses while having fewer side effects than chemotherapies and other drugs that directly destroy cancer cells. Therapeutic agents aiming to stimulate or increase the naturally ability of immune system to kill cancer cells, which often diminishes as the disease progresses, are used in cancer immunotherapy (Figure 1) [4]. Immunotherapy, which attempts to use the host’s adaptive and innate immune responses to achieve long-term eradication of diseased cells, can be broadly classified as passive or active [7].
Figure 1.
Schematic illustration showing the cancer immune response, interventional therapies and its delivery modalities.
The relationship between intestinal microbes and the immune system is mutual in developing tolerance against symbiotic bacteria and antigens present in the food. This makes the immune system prepare innate and acquired immunity against invading microorganism. There needs to be a balance between recognizing gut microbiota and invading pathogenic microbes. The metabolites produced by the microorganism in the gut can alter the balance of inflammatory cytokines in the body and disrupt the production of T cell subsets [8]. Recent research studies elucidate the relationship between gut microbiota and their function in cancer immunotherapy. In one of the studies with an immune checkpoint blockade targeting CTLA-4 and PD-1 using a mouse model, they showed that the gut bacteria have influence in the response tp cancer immunotherapy [9,10]. In another study, the ingestion of the bacteria Bacteroides fragilis with Bacteroides thetaiotaomicron or Burkholderia cepacian increased Th1 response and DC maturation, subsequently enhancing the efficacy of anti-CTLA-4 therapy [9]. Similar experiments conducted with immunotherapy using anti-PD-1 or anti-PD-L1 treatments showed the involvement of gut bacteria in modulating treatment outcomes [11,12,13]. In patients receiving PD-1 medication, the diversity of gut bacteria such as Clostidiales, Ruminococcacease, and Faecalibacterium are increased. Studies have found that the correlation between gut microbiota with respect to immunological profiling in the tumor microenvironment has demonstrated that cytotoxic T cell marker expression was augmented with antigen presentation and processing in patients having favorable gut microorganisms when compared to patients with unfavorable gut microbes [14]. Finding out the gut microbiota which have positive correlations with anti-cancer therapy can boost the efficacy and help patients to benefit from these therapies.
Immunotherapy is fundamentally changing the clinical cancer treatment landscape. It outperformed standard-of-care therapy in several cancer types, including malignant melanoma and lung cancer, resulting in a number of cases with remarkable outcomes, such as total regression of advanced-stage (metastasized) tumors and prolonged disease-free survival [15]. In addition to immune checkpoint inhibitors, which are primarily used for solid tumors, effective cancer immunotherapy has also been achieved through the use of chimeric antigen receptor (CAR) T cell therapies, which have thus far been primarily used to treat hematological malignancies [16]. In the last decade, the US FDA has approved several immunotherapy modalities for treatment, including five immune check point inhibitors, six CAR-T cell therapies, and one oncolytic virus therapy (Table 1).
Table 1.
Cancer Immunotherapy Products on the Market Approved by the US FDA [17].
| Product Name | Therapy | Type | Cancers Approved | Approved Year |
|---|---|---|---|---|
| Roferon-A | Recombinant IFNα2a | Cytokine | Hairy cell leukemia, follicular lymphoma, melanoma, Kaposi sarcoma | 1986 |
| Intron-A | Recombinant IFNα2b | Cytokine | Hairy cell leukemia, follicular lymphoma, melanoma, Kaposi sarcoma | 1986 |
| Aldesleukin | Recombinant IL-2 | Cytokine | Melanoma and kidney cancer | 1992 |
| Sipuleucel-T | Autologous PBMCs activated with Recombinant human PAP–GM-CSF |
Cell-Based Cancer Vaccine | Prostate cancer | 2010 |
| Ipilimumab | CTL A4 mAb | ICI | Melanoma | 2011 |
| Nivolumab | Anti PD-L1 (PD-L1 mAb) | ICI | Melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction cancer | 2014 |
| Pembrolizumab | Anti PD-L1 (PD-L1 mAb) | ICI | Melanoma, lung cancer, head and neck cancer, Hodgkin lymphoma, stomach cancer, cervical cancer, and certain types of breast cancer. | 2014 |
| T-Vec (Talimogene laherparepvec) | GE Oncolytic HSV1 with GM-CSF | Oncolytic Virus | Melanoma | 2015 |
| Atezolizumab | Anti PD-L1 (PD-L1 mAb) | ICI | Urothelial carcinoma, non-small cell lung cancer (NSCLC), triple-negative breast cancer, small cell lung cancer, hepatocellular carcinoma, and alveolar soft part sarcoma. | 2016 |
| Tisagenlecleucel | CD19-specific CAR-T cells | Adoptive cell therapy | B cell acute lymphocytic leukemia and non- Hodgkin lymphoma |
2017 |
| Axicabtagene ciloleucel | CD19-specific CAR-T cells | Adoptive cell therapy | Large B cell lymphoma | 2017 |
| Brexucabtagene autoleucel | CD19-specific CAR-T cells | Adoptive cell therapy | Mantle cell lymphoma (MCL) and acute lymphoblastic leukemia (ALL) | 2020 |
| Lisocabtagene maraleucel | CD19-specific CAR-T cells | Adoptive cell therapy | B cell non-Hodgkin lymphoma | 2021 |
| Idecabtagene vicleucel | B cell Maturation antigen (BCMA) | Adoptive cell therapy | Multiple myeloma | 2021 |
| Ciltacabtagene autoleucel | BCMA | Adoptive cell therapy | Multiple myeloma | 2022 |
| Opdualag | PD1 blocking and Anti-LAG-3 | ICI | Melanoma | 2022 |
2. Cancer Immunotherapy Types
2.1. Checkpoint Inhibitors
Check point inhibitors are the most extensively studied cancer immunotherapy modalities. CTLA4 inhibition and PD-1/PD-L1 blockade are the two most commonly used check point inhibitors. Check point inhibitors regulate the immune response to abnormal cells while protecting healthy tissues from immune attack [18]. T cells are activated to express PD-1 in response to inflammation in TME, which in turn make it possible to detect cancer cells [19]. Cancerous cells expressing PD-L1 render the T cells inactive by binding to them to avoid an immune response. Using checkpoint inhibitory monoclonal antibodies targeting PD-1 or PD-L1 to manipulate this phenomenon, T cells can be effectively used to counter cancerous cells [20,21]. CTLA4, a co-inhibitory molecule that regulates the T cell activation, has another check point inhibitor mechanism. The co-stimulatory molecule CD28 and its ligands CD80 and CD86 are important for the activation, proliferation, and survival of T cells. CTLA-4 blocks CD28 signaling by binding to its ligand CD80 or CD86 and thus inhibits proper T cell response. Thus, antibody against CTLA-4 is used as checkpoint inhibitor to activate T cells’ immune response [22]. The impact of PD-1, PD-L1, or CTLA4 checkpoint inhibitors has been regarded as one of the more efficient antitumor strategies than chemotherapeutics [23].
Cancer immunotherapy clinical trials are being planned in conjunction with check point inhibitors and chemotherapies or other agents [24]. Still, checkpoint inhibitors are not obsolete, and there are some limitations to them, such as severe side effects to certain organs when checkpoint inhibitors are systemically administered [25,26]. Still, clinical research studies are being conducted to determine the underlying mechanism of checkpoint inhibitors and their limitations in dealing tumor mechanisms [27].
2.2. Cytokines
Recombinant IFN was the first cytokine to be approved for immunotherapy in 1986. Since then, interferons, interleukins, and granulocyte macrophage colony-stimulating factor (GM-CSF) have been studied clinically for their immunotherapy potential [28]. Interferons are produced in response to microbial pathogen-induced immune activation, which results in the activation of macrophages, natural killer (NK) cells, dendritic cells, and lymphocytes. Interferon activates immune cells in the tumor microenvironment, inhibiting angiogenesis [29]. Interleukins stimulate the activity and growth of T cells, specifically CD4+ and CD8+ cells. There is a number of interleukins that have pro and antitumor activity (IL-4) depending on the tumor type, stage and location and type of cells which produce them. IL-2 is important for T cell proliferation, whereas IL-10 inhibits T cells activation [30]. GM-CSF stimulates the immune system through two mechanisms: it promotes T cell homeostasis and dendritic cell differentiation, which results in the production of tumor-specific antigens. GM-CSF can also help granulocytes’ recovery after chemotherapy [31]. Currently, extensive research is being conducted to reduce the adverse effects of individual therapies by combining checkpoint inhibitors with cytokines or chemotherapies [32].
2.3. Vaccinations
Tumor cell lysate, dendritic cells, and nucleic acids are examples of cancer vaccines [33,34,35]. Dendritic vaccines are the most extensively researched cell-based vaccines [36]. Autologous dendritic cells are collected from patients and engineered so that they express tumor-associated antigens, activating T cells to attack the tumor [37]. Sipuleucel-T is one of the approved dendritic cell vaccines for the treatment of prostate cancer that was approved in 2010 for its ability to successfully prolong patient survival [38]. Manipulating dendritic cells to express targeted antigens and induce T cells against tumor can improve the efficacy and potency of dendritic vaccines [39].
Nucleic acid therapeutics such as DNA- or RNA-based vaccines are emerging as alternatives to conventional vaccines [40]. Nucleic acid vaccines must be delivered intracellularly to the target cells, where they are translated to induce antigen expression. These antigens are presented to T cells in order to activate them against cancer cells. Recently, mRNA vaccines have gained attention because they have more advantages than DNA vaccines, such as the ability to extend the half-life of mRNA with minor modifications. However, mRNA is prone to degradation by nucleases, so it requires a transfection reagent or delivery technologies for intracellular delivery [41].
Vaccines are based on neoantigens that can boost the immune response against cancer cells [42,43]. Because of genetic instability, gene mutations occur in the coding region during carcinogenesis, resulting in the formation of proteins that are not present in normal cells. By activating the immune system, these proteins can be targeted specifically against cancer cells. Delivery methods must be designed in such a way that they increase the stability and protection against cancer [44].
2.4. Antibodies That Are Agonistic
Agonistic antibodies are specifically designed to bind to T cell receptors and activate intracellular signaling pathways in order to effectively combat cancer cells. Monoclonal antibodies (mAbs) targeting immune checkpoints such as CTLA-4 and PD1/PD-L1 have recently been developed for antitumor activity [45]. Agonist mAbs developed against the CD40 immune receptor can increase the tumor-infiltrating T cells (TILs), which can effectively eliminate cancer cells [46].When CD40 interacts with CD40 ligand in dendritic cells, it activates specific T cells, triggering a cascade of antitumor responses [47]. Agonist antibody-based clinical trials are currently being conducted against various receptors, targeting 4-1BB, OX40, and CD 27, but due to their toxicity, alternate delivery methods are required to mask their toxicity potential without compromising antitumor activity [48,49,50,51].
2.5. T Cells with Alternations
Following successful clinical trials and FDA approval, T cell engineering has recently gained attention. Autologous T cells were collected from cancer patients’ blood and genetically engineered to express chimeric antigen receptors found on tumor cells but not on healthy cells in the CAR-T cell approach. CAR-T cells recognize the target antigen on tumors and induce tumor cell death when re-engineered T cells are infused back into the patient [52,53]. The advantage of CAR-T cell therapy is that it is a single infusion therapy that can provide protection for up to a decade after injection [54]. CAR-T therapy has its own drawbacks, such as the fact that it is technically complex, time-consuming, and expensive to produce, which has been a concern in the implementation of CAR-T-based therapies [55]. CAR-T cells were unable to penetrate and interact with antigen receptors in certain solid tumors and complex TME, necessitating the use of combination therapies to improve the efficacy of CAR-T cell therapy [56,57].
Since 2017, the US FDA has approved six CAR-T therapies for blood cancers such as lymphoma, certain leukemias, and, most recently, multiple myeloma. CD-19 is a target antigen for B cell acute lymphoblastic leukemia (ALL), B cell non-Hodgkin lymphoma, follicular lymphoma, mantle cell lymphoma (MCL), and B cell maturation antigen (BCMA) targeting against multiple myeloma [58]. T cell receptor (TCR) T cells are T cells isolated from patients and genetically engineered to express specific peptides and human leukocyte antigens (HLA), resulting in TCR-T cells that are recognized by tumor-associated antigens and effectively kill tumor cells [59]. TCR-T cells, unlike CAR-T cells, are MHC-dependent, so they must be matched with the patient after genetic engineering, which is critical in TCR-T cell cases [60]. Both CAR-T cells and TCR-T cells require further development to improve their applicability with solid tumors while minimizing the associated side effects and toxicity.
2.6. Virotherapy with Oncolytic Agents
Oncolytic viruses have shown promise in the treatment of cancer. Specific viruses that can replicate in the cancer cells induce antitumor immune attacks in the tumor [61,62]. Viruses have been genetically modified to attack and destroy tumor cells while leaving normal cells alone [63,64,65]. Oncolytic viruses used against cancer immunotherapies include adenovirus, vaccinia virus, herpes simplex virus, measles virus, Reo virus, Newcastle disease virus, Coxsackie virus, vesicular stomatitis virus, and Pseudovirus [66]. Antitumor enhancement is achieved in oncolytic adenovirus CG0070 by expressing GM-CSF against bladder cancer [67]. David Ruano et al. showed that the combined treatment of oncolytic adenovirus ICOVIR-5 with mesenchymal stem cells resulted in disease stabilization in neuroblastoma patients, according to a first-in-human and child study [68]. Kim et al. studied several genetically modified vaccinia viruses. In a liver and lung model, deletion of thymidine kinase and expression of GM-CSF prevented metastases [69]. Yoo et al. demonstrated that a vaccinia virus lacking thymidine kinase effectively suppressed stem cell-like colon cancer cells [70]. In addition, they demonstrated that the engineered vaccinia virus can effectively eradicate metastatic liver cancer cells in another study [71]. T-VEC, an oncolytic herpes simplex virus engineered to secrete GM-CSF, was recently approved by the US Food and Drug Administration to treat advanced melanoma [72].
3. Administration Mode
The route of administration (ROA) of a drug can affect its therapeutic efficiency during the delivery process [73,74,75] ROA is an important consideration when developing the delivery immunotherapy delivery methods for a specific tumor treatment. When compared to non-target routes of administration such as systemic administration, directly injecting drugs into tumors (intratumoral) can elucidate better efficacy in terms of antitumor effect [76]. Intratumoral injection directly into the tumor is possible for accessible tumors, but for tumors that are not easily accessible, other modes of administration must be used to effectively deliver drugs to the tumors (Table 2) [77]. The therapeutic efficacy is proportional to the control-release mechanism, which affects how the payload drug is transported in the appropriate medium to comprehend the microenvironment. Understanding the tumor’s microenvironment and the accessibility for the drugs in order to effectively deliver the drugs is a challenge that must be considered. Innovative technologies for effectively delivering drugs for cancer immunotherapy are being developed.
Table 2.
Route of administration for cancer immunotherapy.
| Route of Administration | Advantages | References |
|---|---|---|
| Oral Administration |
|
[78,79,80] |
| Intravenous Administration |
|
[81,82,83] |
| Subcutaneous Administration |
|
[84,85,86] |
4. Cancer Immunotherapy Delivery Methods
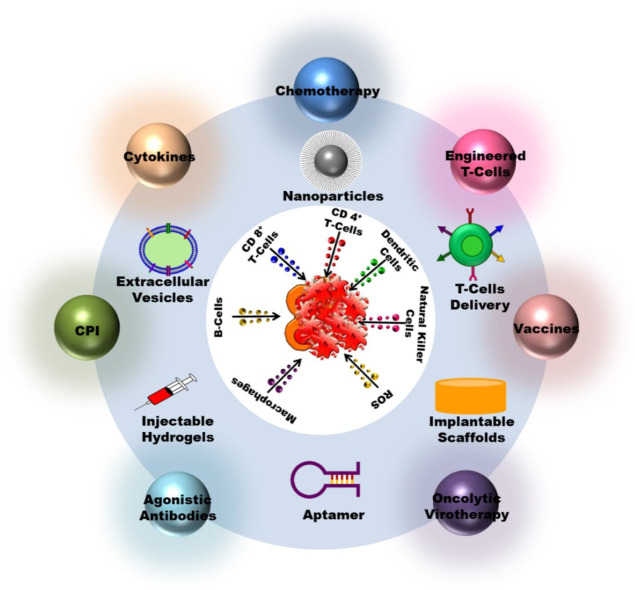
Successful cancer immunotherapy necessitates efficient and effective delivery methods, as well as drug efficacy that is specific and less toxic to host cells (Figure 2). The difficulties in treating a cancer with a drug or biomaterial range from dosage, formulation, homing, degradation, and delivery, all of which must be taken into account when designing a treatment. When developing a drug and its delivery methods, biological and physiochemical parameters should be taken into account. This section discusses the various methods for delivering effective cancer immunotherapy treatments (Table 3).
Figure 2.
Efficient and effective delivery methods specific and less toxic to host cells used in cancer immunotherapy.
Table 3.
Delivery techniques for cancer immunotherapy.
| Delivery Technology | Types/Source | Cargo | Cancer Type | Reference |
|---|---|---|---|---|
| Nanoparticles | Liposomes | ErbB2/HER2 peptide | Renal carcinoma | [87] |
| OVA | Thymoma | [88] | ||
| ACT-cell-specific antibodies and Interleukin-2 (IL-2) | Melanoma | [89] | ||
| Plasmid encoding telomerase-specific oncolytic adenovirus | Colorectal cancer | [90] | ||
| Polymer | OVA and Hydroxychloroquine | Thymoma | [91] | |
| PLK1 inhibitor and PD-L1 antibody, | NSCLC | [92] | ||
| IR780 and PD-L1 antagonist | Colorectal cancer | [93] | ||
| Dendrimer | PD-L1 siRNA and IL-2 encoding plasmid DNA | HCC | [94] | |
| Inorganic nanocarriers | Vesicular stomatitis virus, | Colorectal cancer | [95] | |
| Adenovirus | Pancreatic cancer, Colorectal cancer | [96] | ||
| mRNA-encoding OVA and R848 | Melanoma | [97] | ||
| RNA/DNA Technology | Anti-PD-1 antibody and CpG oligodeoxynucleotides, | Melanoma | [98] | |
| OVA | Melanoma | [99] | ||
| Exosomes | Let-7a miRNA | Breast cancer | [100] | |
| EGFR nanobodies | Epidermal | [101] | ||
| Cisplatin | Ovarian cancer and Hepatocarcinoma | [102] | ||
| Nanovaccine | Peptide neoantigen (Adpgk) and R848 and CpG | Colorectal cancer | [103] | |
| cyclic dimeric guanosine monophosphate (CDG) | melanoma | [104] | ||
| Extracellular Vesicles | Dendritic cells | VEGF siRNA | Breast cancer | [105] |
| Bone Marrow-Derived MSC | TRAIL | lung Cancer | [106] | |
| A549 Lung Carcinoma ells (Human) | Doxorubicin | Lung carcinoma | [107] | |
| B16-F10 melanoma cells (Mouse) | CpG DNA | Melanoma | [108] | |
| H22 Hepatocarcinoma cells (Mouse) | Doxorubicin, 5-FU | Hepatocarcinoma | [109] | |
| Implantable Scaffolds | Collagen and HA cross-linking scaffold | GEM, poly(I:C) | Breast cancer | [110] |
| PLG scaffold | GM-CSF, CpG-ODNs | Melanoma | [111] | |
| Hyaluronic acid scaffold | CAR-NK cells | Breast cancer | [112] | |
| Injectable Scaffolds | Alginate Hydrogel | Celecoxib, PD-1 antibody | Melanoma, Breast cancer | [113] |
| PEGylated poly(L-valine) hydrogel | TCL, poly(I:C) | Melanoma | [114] | |
| ROS-degradable hydrogel | GEM, PD-L1 antibody | Melanoma, Breast cancer | [115] | |
| Cell-Based Delivery | Erythrocyte | Curcumin | Liver cancer | [116] |
| Glucose oxidase, Tirapazamine | Colon cancer | [117] | ||
| DOX | Lymphoma | [118] | ||
| Cytotoxic T cells | Taxol | Gastric cancer | [119] | |
| NK cell | TCPP | Breast cancer | [120] | |
| Car-T Cells | Glioblastoma, hepatic colorectal metastases, peritoneal carcinomatosis, pleural mesothelioma, mesothelioma | [121] |
4.1. Nanoparticles
Nanomaterials are advantageous in several parameters, including surface-to-volume ratio, photo dynamics, magnetic and electrical conductivity, optical absorption, and fluorescent behavior properties, which make them an effective additive in cancer immunotherapy [122]. Recent technological aspects of nanoparticles have sparked interest in the use of nanomedicine-based drug delivery systems because they can potentially cross biological barriers, have biocompatibility, drug transport, and provide sustained drug release in cancer immunotherapy approaches [123]. To overcome the barriers to drug deliver to the tumor microenvironment, a powerful delivery platform that penetrates the complex structure surrounding the tumor is required [124]. The use of nanoparticles in drug delivery is one of the promising novel methods in the application of cancer immunotherapy [125].
Nanoparticle-based approaches to drug delivery drugs to tumors have attracted the interest of researchers because they are cancer cell target specific [126]. Nanoparticle-based delivery that directly targets the tumors can improve drug biodistribution and localization within the tumor [127]. Small molecules, proteins, peptides, antibodies, cytokines, and monoclonal antibodies can be delivered by nanoparticles using a variety of platforms, including liposomes, polymers, inorganic nanocarriers, dendrimers, and exosomes [128]. One of the most important properties for a nanoparticle in cancer immunotherapy is enhanced permeability and retention (EPR), which determines the drug’s accumulation time in the tumor microenvironment [129]. The use of tumor-associated antigens (TAA) to direct the immune system against cancerous cells results in less antitumor activity. Combining them with a nanodelivery system effectively protects them from degradation and allows them to interact with antigen-presenting cells, resulting in the stimulation of cytotoxic T lymphocytes with an effective antitumor mechanism [130].
Deng et al. used NK cell-masked nanoparticles, which can be activated by photodynamic therapy to attack the cells and induce immunogenic cell death (ICD), releasing damage-associated molecular patterns, as well as NK-coated cells targeting M1 macrophages, which eventually promotes antigen-presenting cells’ (APCs) maturation, leading to T cell activation and elimination [120]. Cancer cells were eradicated using novel photoimmunotherapy-based nanoparticles. By synthesizing an apoferritin nanoparticle protein cage as a photosensitizer conjugated with fibroblast activation protein specific antibody, Zhen et al. were able to effectively bind the fibroblasts in the tumor region, photoirradiation modulated the antitumor immune response [131]. Another strategy for inducing immune system against cancer is to manipulate nanoscale bioconjugates. One such strategy is the use of Halloysite nanotubes coated with polyethylene glycol, functionalized with folate residues and loaded with doxorubicin, a chemotherapeutic drug against 4T1-bearing mice, which demonstrated 65% tumor growth inhibition compared to 35% for doxorubicin alone [132].
Copolymer micelles have been shown to have EPR effects when used to target tumors. Grafting polylactic acid onto carboxymethyl cellulose as a copolymer and functionalizing with anti-EpCAM antibody can be used for doxorubicin chemotherapeutic drug delivery against hepatic cells (HepG2). Doxorubicin drug release was in specifically at the tumor site, and functionalized drug-loaded micelles exhibited antitumor effects in both in vitro and in vivo conditions [133]. Chiang et al. demonstrated that a combination of anti-PDL1 checkpoint inhibitors and T cell activators conjugated to superparamagnetic iron oxide nanoparticles and functionalized fucoidan–dextran forming IO@FuDex3 nanocomplexes were capable of activating immune cells and neutralizing tumors in a 4T1 breast cancer mouse model [134]. Badrinath et al. demonstrated antitumor efficacy by enhancing apoptosis by combining an oncolytic vaccinia virus with poly lactic-co-lactic glycolic acid nanofiber as a delivery method against colon carcinoma [135]. Another method for targeting tumors is to use magnetic nanoparticles against tumors through various techniques such as manipulating the tumor environment, activating APCs, macrophage polarization, T cell stimulation, and NK cell delivery [136].
Nano vaccines are intended to contain tumor-specific antigens as well as TAA in order to suppress the tumor. Nano vaccines target antigens or components found exclusively in tumors or expressed in tumors. APCs such as macrophages and dendritic cells will come into contact with vaccine antigens [137]. Cell-, virus-, peptide-, DNA-, and mRNA-based vaccines have been shown to be effective in treating a variety of cancers. The main advantage of using nanoparticles is that they can be designed to produce an effective immune response against cancers based on the target cells [138].
Jin et al. demonstrated an in situ cancer vaccine-based approach; in their study, they designed in situ vaccines by combining two synergetic approaches. First, ferrimagnetic nano cubes were encapsulated into an amphiphilic polymer, which generates the antigens by a magnetic field and destroys the primary tumor, and another polymeric nanoparticle coated with adjuvant R848 (resiquimod) delivers the formed antigens to the lymph node, activates the APCs and creates an antitumor immune response to distant tumors [139]. Li et al. conducted another in situ based study. The formed TAAs were captured and delivered to APCs by photodynamic therapy, effectively eliminating cancer cells synergistically with checkpoint therapy [140].
In another study, fluoropolymer combined with antigen ovalbumin aided dendritic cell maturation and antigen presentation, leading to tumor suppression. When these fluoropolymers were combined with antigens from resected cell membranes from primary tumors, it resulted in inhibition of tumor recurrence and metastasis [141]. Luo et al. demonstrated the efficacy and abscopal effect of neoantigen-based immunotherapy against colon carcinoma and melanoma where nano vaccines inhibited tumor growth and survival rates in an in vivo model [142].
Organic and inorganic nanoparticles were combined in various ways to create effective photothermal agents to debilitate cancer cells [143,144]. Because of their biocompatibility and optical properties, gold nanoparticles were a candidate for nanoparticle synthesis, but their poor photothermal therapy application prompted a modification in their surface with silica; a silica-coated gold nanoparticle cluster was shown to displau effective photothermal transduction against prostate cancer cells in vitro and the tumors completely disappeared after 15 days [145]. Another method of inducing an immune response against cancer is to use nanometal organic frameworks (MOFs) loaded with anti-DEC205 antibody. Sonodynamic immunotherapy was used in this study, in which ultrasound-based deep-tissue-penetrating sonication functionalized the AMR-MOF@AuPt, resulting in large amount of reactive oxygen species that eliminated cancer cells and distant metastases [146].
Many strategies have been employed in attacking the TME using nanoparticles; one such strategy is to target the fibroblast cells associated within the tumor environment. Cancer-associated fibroblasts (CAF) were targeted using various nanoparticle delivery methods, and were able to be delivered into the deeper stroma reducing α-SMA (smooth muscle actin) levels around the tumor tissue and subsequently destroying the cancer cells [147,148,149,150]. Recently, studies have found that the macrophages are a double-edged sword; one could polarize macrophage into a tumor-suppressing subtype (M1) by exposing them to IFN-γ and lipopolysaccharides to produce IL-12, which arrests the tumor growth. Regulating tumor-associated macrophages (TAM) using iron oxide nanoparticles could polarize M2 macrophage into M1 macrophage [120,151,152,153]. One another strategy to target TME is by modulating the tumor extracellular matrix (ECM), as it provides support and regulates cellular activities and can be a targeting source to hamper the tumor growth using nanoparticles. Laminin in the ECM could be used as a target by designing laminin-mimicking, self-assembling peptides to form a nanoparticle, which would prolong retention time and accumulate at the tumor site and inhibit metastasis of cancer [154]. Other studies focus on components prevailing in ECM such as collagen, hyaluronic acid, matrix metalloproteinases, which are targeted using various nanoparticle techniques to suppress tumor growth and metastasis [155,156,157,158]. Nanoparticle-based strategies are used against vasculatures as they provide growth factors, nutrients and play an essential role in growth of the tumor; nanoparticles carrying anti-angiogenic drugs effectively inhibit angiogenesis and metastasis [159,160,161,162]. These nanoparticle-based strategies open new insights for cancer immunotherapy and can translate into clinical treatment for personalized therapy.
4.2. Vesicles Extracellular
Extracellular vesicles are small membrane vesicles formed by fusion of the plasma membrane and endosomes that are secreted by cells [163]. As everyday research reveals their potential in delivering drugs to cancer cells, EVs are emerging as a drug delivery technology [164,165]. EVs are complex membrane vesicles that travel through tight junctions to selectively enter cells [166]. Zitvogel et al. discovered the exosomes can be derived from dendritic cells with functional MHCs and tumor antigens on the surface, leading to tumor neutralization by cytotoxic T lymphocytes (CTL) [167].
Dendritic cell-derived exosomes increased NK cell antitumor activity in a clinical trial against non-small cell lung cancer (NSCLC) as maintenance immunotherapy for patients undergoing chemotherapy [168,169]. Wang et al. used exosomes to deliver drugs to a tumor in a liver mouse model. PTX, which has a low therapeutic efficacy due to its poor solubility, was packaged into exosomes to increase its potential and showed higher efficacy in tumor retention and inhibition [170]. Curcumin-loaded exosomes were used in another in vivo mouse model study to successfully cross the blood–brain barrier and deliver the drug against malignant glioma in the brain [171].
4.3. Biomaterials
Implantable functional scaffolds are frequently used in cancer immunotherapy to reprogram the biological responses by delivering bioactive chemicals or cells in a controlled manner [172]. Biomaterial-based delivery systems have properties such as minimal invasiveness, targeted delivery, controlled release, high efficacy, immune cell activation, and low toxicity, making them a potential cancer immunotherapy technique [135,173]. Nanomaterials and scaffold-based biomaterials are commonly used as implantable and injectable biomaterials to elicit immune responses and thus antitumor activity [174].
Long-term stimulation of APCs was achieved by constructing a 3D microporous alginate-reduced graphene oxide (rGO) scaffold loaded with GM-CSF, ovalbumin, and cytosine–phosphate–guanine oligonucleotides. The rGO component of the implantable scaffold’s large surface area and hydrophobic surface allow for significant loading and a very gradual release of a loaded antigen. In a B16 melanoma tumor model in mice, the scaffold recruited dendritic cells, which then activated T cells, effectively suppressing the tumor [175]. Another study found that loading a blood clot scaffold with liposomal nanoparticles containing both vaccine and siRNA can effectively induce DCs, leading to T cell activation and tumor suppression in various tumor and mouse models [176].
A cancer vaccine composed of whole tumor lysate-based antigens and nanoadjuvants expressing Toll-like receptor (TLR) 3 agonists, as well as gemcitabine as an MDSC-depleting agent, was shown to improve antitumor immunity by lowering immune suppression in the tumor microenvironment [110]. Similarly, Ren et al. used a degradable and regulatable macroporous implantable scaffold using methacrylate hyaluronic acid loaded with three different compounds, the chemotherapeutic medication PTX, APCs activator TLR7 agonist (R837), and immune checkpoint blockade molecules, which was then implanted in a 4T1 breast tumor mouse model, and showed depletion of myeloid-derived suppressor cells (MDSC) and M2 macrophages, enhancement of APCs, and increased antitumor immunity [177]. Ahn et al. created a 3D-engineered hyaluronic acid scaffold that increased mRNA expression, cytokine release, and tumor lysis, resulting in improved antitumor efficacy for a resected breast cancer model [112].
Because each type of biomaterial has distinct advantages in certain contexts, the choice of biomaterial design, whether injectable or implantable, is fundamentally driven by application requirements. Injecting implantable materials directly into the organs or tissues is a much less invasive procedure than surgical implantation, and it reduces the risk of tissue damage and the inflammatory response associated with wounds [172]. To make injectable biomaterials, hydrogels, cryogels, and self-assembling systems can be made from a variety of natural and synthetic ingredients [178]. Liu et. al, created a supramolecular hydrogel for locoregional delivery that functions as both an ICD and immune checkpoint inhibitor therapeutic [179].
In another study, they created an intelligent drug delivery system with controlled and sustained drug release. They created in an injectable nanofiber hydrogel by combining betamethasone phosphate and calcium ion with anti-programmed cell death protein ligand 1 antibody (αPDL1), which results in cross-linking filamentous assemblies. By blocking the NF-B signaling pathway, the anti-inflammatory steroid betamethasone phosphate has been incorporated into an injectable nanofiber hydrogel to reprogram the protumoral immunosuppressive TME, and the sustained release of PDL1 from the hydrogel stimulates the T cells to synergistically increase the immunological response of tumor cells [180]. Another strategy is co-delivery, which combines a hydrogel with a tumor vaccine and immune checkpoint inhibitors to improve the therapeutic efficiency against melanoma and 4T tumors [181].
4.4. T Cell Therapy Delivery Methods
The advancement of clinical grade bench-to-bedside technology for isolating, genetically engineering, and ex vivo expansion of T cells from one’s own patient blood has brought T cell-based therapies to the forefront of cancer immunotherapy [55]. Tumor-infiltrating lymphocytes (TILs) and T cell receptor manipulation results in expressing specific antigens and HLA to effectively eliminate tumor cells [182].
Adoptive cell therapy is one such T cell therapy that is effective in treating blood cancers. Chimeric antigen receptor-T cells (CAR-T) are one such therapy that has recently received several FDA approvals, and products in the US, for which patient blood is collected and T cells are engineered to treat a variety of B-cell malignancies. Although this technology is effective for blood cancers, its limited effectiveness against solid tumors due to poor infiltration against the complex tumor microenvironment has prompted researchers to look for a delivery system that will allow CAR-T cells easier access to cancerous cells [183]. Using injectable or implantable bio scaffolds for locoregional delivery has been successful, and codelivery of CAR-T cells with immunostimulatory molecules has improved long-term delivery into the tumor microenvironment. For instance, Grosskopf AK et al., in a mouse model, used a polymer-nanoparticle hydrogel (PNP) to deliver CAR-T cells with IL-15, both proximal and distal to tumors, potentially accessing solid tumors and curing them [184].
Combination therapy in one or more modalities can have a synergistic effect on the treatment of solid tumors. Hu et al. carried out one such study where they used CAR-T cells in combination with immune checkpoint inhibitors in a melanoma mouse model. Biodegradable hydrogel encapsulates CAR-T cells, targeting human chondroitin sulfate proteoglycan 4 (CSPG4.CAR) with nanoparticle-coated IL-15 and anti-PDL1 conjugated with human platelets in combination, allowing IL-15 to activate and proliferate CAR-T cells while blocking the PD1/PDL1 pathway to eradicate tumor cells [185]. Various delivery modalities have been used to effectively deliver and improve the access of CAR-T cells to solid tumors. Biomedically designed polymeric devices can provide effective access to incompletely resected or inoperable tumors, and the conjunction of soluble biomolecules and T-cell activation antibody ligands can achieve the multi-faceted promotion of antitumor activity against cancerous cells. 3D bio scaffolds such as polymerized alginate-collagen mimetic peptide matrices aided T cell migration to the tumor site, as did combining porous silica microparticles into matrices capable of encapsulating and releasing biomolecules for extended periods of time [186].
With new delivery technologies emerging for cancer immunotherapy, one has to carefully choose the appropriate method by which drug efficiency can be improved. Some of the delivery technologies are listed below with their advantages and disadvantages for cancer immunotherapy (Table 4).
Table 4.
Advantages and disadvantages of delivery techniques in cancer immunotherapy.
| Delivery Modalities | Immunotherapy Classes | Advantages | Disadvantages |
|---|---|---|---|
| Nanoparticles |
|
|
|
| Extracellular Vesicles |
|
|
|
| Implantable Scaffolds |
|
|
|
| Injectable Scaffolds |
|
|
|
| Cell-Based Delivery |
|
|
|
5. Clinical Trails and Patents
Clinical trials are conducted using new delivery systems for cancers. To analyze, clinically translate and market personalized medication, rigorous research is required. Some of the clinical trials that have been studied to determine their effectiveness and safety are shown in below in Table 5.
Table 5.
Current clinical trials for cancer immunotherapy by various delivery technologies.
| Clinical Trial Identifier | Phase | Treatment | Therapy | Delivery Modalities | References |
|---|---|---|---|---|---|
| NCT00466960 | II | Sargramostim and Paclitaxel Albumin-Stabilized Nanoparticle Formulation in Treating Patients With Advanced Ovarian Cancer, Fallopian Tube Cancer, or Primary Peritoneal Cancer That Did Not Respond to Previous Chemotherapy | Combined Therapy (Chemotherapy and Cytokine) | Nanoparticle | [187] |
| NCT02410733 | I | Evaluation of the Safety and Tolerability of i.v. Administration of a Cancer Vaccine in Patients with Advanced Melanoma (Lipo-MERIT) | Vaccine | Liposome | [188] |
| NCT01753089 | I | Dendritic Cell Activating Scaffold in Melanoma | Cell Therapy | Scaffold | [189] |
| NCT00103506 | III | Study of DOXIL/CAELYX (Pegylated Liposomal Doxorubicin) and VELCADE (Bortezomib) or VELCADE Monotherapy for the Treatment of Relapsed Multiple Myeloma | Chemotherapy | Liposome | [190] |
| NCT02379845 | II/III | NBTXR3 Crystalline Nanoparticles and Radiation Therapy in Treating Randomized Patients in Two Arms with Soft Tissue Sarcoma of the Extremity and Trunk Wall | Radiotherapy | Nanoparticle | [191] |
| NCT01052142 | I | Safety Study of a Liposomal Vaccine to Treat Malignant Melanoma | Vaccine | Liposome | [192] |
| NCT00157209 | IIb | Phase 2b Randomized Controlled Study of Tecemotide (L-BLP25) for Immunotherapy of NSCLC (Non-Small Cell Lung Cancer) | Vaccine | Liposome | [193] |
| NCT00924326 | I/II | CAR T Cell Receptor Immunotherapy for Patients With B-cell Lymphoma | CAR-T | [194] | |
| NCT01454596 | I/II | CAR T Cell Receptor Immunotherapy Targeting EGFRvIII for Patients with Malignant Gliomas Expressing EGFRvIII | CAR-T | [195] | |
| NCT01865617 | I/II | Laboratory Treated T Cells in Treating Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia, Non-Hodgkin Lymphoma, or Acute Lymphoblastic Leukemia | CAR-T | [196] |
Cancer drugs require novel delivery systems to make them effective and safe therapies. Recent anti-tumor therapies are designed in such a way they are efficacious in dealing complex tumor environment. Listed below in Table 6 are some of the innovative discoveries to combat cancer using various delivery techniques.
Table 6.
Novel patents for cancer immunotherapy by various delivery technologies.
| Patent Number | Inventors | Title |
|---|---|---|
| US20090010948A1 | Fang Ping Huang, Yu Xiao Chen, Kwan Man | Anti-tumor vaccines delivered by dendritic cells devoid of interleukin-10 |
| US20040156846A1 | Wolfgang Daum, Gerald DeNardo, Diane Ellis-Busby, Alan Foreman, Douglas Gwost, Erik Handy, Robert Ivkov | Therapy via targeted delivery of nanoscale particles using L6 antibodies |
| WO2017151727A1 | Zhen GU, Chao Wang, Yanqi YE | Enhanced cancer immunotherapy by microneedle patch-assisted delivery |
| US20160361268A1 | Chih-Peng Liu, Ya-Chin Lo, Ming-Cheng Wei, Maggie LU, Shuen-Hsiang CHOU, Shih-Ta Chen, Hsiang-Wen TSENG | Intralymphatic delivery of hyaluronan nanoparticle for cancer metastasis |
| WO2011097384A2 | Dapeng Zhou, Li Chun, Patrick Hwu | Tumor targeted delivery of immunomodulators by nanoplymers |
| US8785371B2 | Rameshwar Patil, Eggehard Holler, Keith L. Black, Julia Y. Ljubimova | Drug delivery of temozolomide for systemic based treatment of cancer |
| US20160346204A1 | Wenbin Lin, Chunbai He, Demin Liu | Nanoscale carriers for the delivery or co-delivery of chemotherapeutics, nucleic acids and photosensitizers |
| US9610250B2 | Tarek M. Fahmy, Eric STERN, Richard A. Flavell, Jason Park, Alyssa Siefert, Stephen H. Wrzesinski | Nanolipogel vehicles for controlled delivery of different pharmaceutical agents |
| US20080044484A1 | Boris Minev | Use of polymeric nanoparticles for vaccine delivery |
| US20040038406A1 | Gretchen Unger, Beverly Lundell | Nanoparticle delivery systems and methods of use thereof |
6. Challenges and Future Progress
New developments in understanding the cancer prognosis and novel therapeutic approaches have called for innovative delivery methods in administering anticancer drugs. Immunotherapy-based drugs are currently studied in various types of cancers; their effect on solid tumors is meager because the low infiltration of immune cells makes lower tumor immunogenicity, leading to an immunosuppressive tumor environment. Developing unique and novel drug delivery systems in combination with multiple cancer therapies would allow the treatment of solid tumors. The key issues such as the controlled release of drugs at the specific site, techniques to assess these delivery mechanisms and their effect on the cellular or molecular level are some of the constraints in developing a robust delivery system. In the past thirty years, cancer nanomedicines-based approaches have achieved progress in tackling the tumor microenvironment by understanding the enhanced permeability and retention, but still certain hurdles in clinically driven transition in developing and approving are needed to be addressed [197].
Many cancer nano formulations have certain drawbacks like off-target accumulation, stability, in vitro to in vivo correlation and fulfilling regulatory norms in bringing clinical translation is of some major issues [198]. Major challenges in developing drug delivery systems using nanoparticle include physiochemical characterization, large-scale production, developing low-toxicity nanoparticles and fulfilling the regulations in their successful release into the market [199]. CAR-T cell therapy has gained interest after breakthrough approvals recently, but still various clinical applications need to be resolved, with better cell engineering and genome-editing technologies to improve the efficacy and safety against various types of cancers. Despite promising results with delivery methods using extracellular vesicles, nanoparticles, scaffolds and cellular-based vehicles to deliver drugs against cancers, more insights into the mechanism of TME to effectively infiltrate and the evade immune system are needed for these treatments to reach their full potential in countering cancers.
A multicentric approach in developing oncological therapeutic research using novel drugs and delivery systems has gained popularity with the advancement of 3D-printing and personalized delivery digital devices. In the future, there will be significant progress in the development of nanorobots or implantable microchips that can deliver drugs and control tumor progress. The future onco-medicine developments require intelligent and robust multi-disciplinary approaches, where computer-based artificial intelligence and biotechnology should go hand in hand in developing intelligent nanorobotic-based drug carriers for delivering nanomedicines [200]. One has to carefully iterate their potential to impact on animals and environment, which needs to be considered before their approval for treatment.
7. Conclusions
Immunotherapy was developed in response to the ever-increasing research on cancer and understanding and the use of technologies to find an effective treatment for cancer. Because cancer is a complex disease, smart and intelligent delivery technologies must be developed to overcome the challenges of controlling its growth and elimination. To achieve successful cancer remission, novel strategies and therapy regimens will be tested in preclinical and clinical research. In this review, we discuss the various cancer treatment approaches that use drugs and biomaterials to exploit the immunological cascades against the tumor microenvironment. Despite limitations and challenges in developing technologies to increase drug delivery or efficacious results, combining one or more therapies with improved delivery technologies can result in an effective clinical translation. Precision targeting approaches with immunologically effective, low-toxicity technologies in cancer immunotherapy and delivery should translate to clinical implications and eventually benefit patients.
Author Contributions
Conceptualization, P.M. and S.Y.Y.; methodology, P.M. and M.R.; validation, H.Y.W. and S.Y.Y.; investigation, P.M. and M.R.; writing—original draft preparation, P.M. and M.R.; writing—review and editing, S.Y.Y.; supervision, S.Y.Y.; project administration, S.Y.Y.; funding acquisition, H.Y.W. and S.Y.Y. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All data needed to support the conclusions are present in the paper. Additional data related to this paper may be requested from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by Biomedical Research Institute Grant (20220033), Pusan National University Hospital.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Yadav D., Kwak M., Chauhan P.S., Puranik N., Lee P.C.W., Jin J.O. Cancer immunotherapy by immune checkpoint blockade and its advanced application using bio-nanomaterials. Semin. Cancer Biol. 2022;86:909–922. doi: 10.1016/j.semcancer.2022.02.016. [DOI] [PubMed] [Google Scholar]
- 2.Benavente S., Sánchez-García A., Naches S., LLeonart M.E., Lorente J. Therapy-Induced Modulation of the Tumor Microenvironment: New Opportunities for Cancer Therapies. Front. Oncol. 2020;10:582884. doi: 10.3389/fonc.2020.582884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanmamed M.F., Chen L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell. 2018;175:313–326. doi: 10.1016/j.cell.2018.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bondhopadhyay B., Sisodiya S., Chikara A., Khan A., Tanwar P., Afroze D., Singh N., Agrawal U., Mehrotra R., Hussain S. Cancer immunotherapy: A promising dawn in cancer research. Am. J. Blood Res. 2020;10:375–385. [PMC free article] [PubMed] [Google Scholar]
- 5.Liu M., Guo F. Recent updates on cancer immunotherapy. Precis. Clin. Med. 2018;1:65–74. doi: 10.1093/pcmedi/pby011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen D.S., Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Naran K., Nundalall T., Chetty S., Barth S. Principles of Immunotherapy: Implications for Treatment Strategies in Cancer and Infectious Diseases. Front. Microbiol. 2018;9:3158. doi: 10.3389/fmicb.2018.03158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan X., Song M., Wang A., Zhao Y., Wei Z., Lu Y. Microbiome Crosstalk in Immunotherapy and Antiangiogenesis Therapy. Front. Immunol. 2021;12:747914. doi: 10.3389/fimmu.2021.747914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vétizou M., Pitt J.M., Daillère R., Lepage P., Waldschmitt N., Flament C., Rusakiewicz S., Routy B., Roberti M.P., Duong C.P. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sivan A., Corrales L., Hubert N., Williams J.B., Aquino-Michaels K., Earley Z.M., Benyamin F.W., Man Lei Y., Jabri B., Alegre M.-L. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti–PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Routy B., Le Chatelier E., Derosa L., Duong C.P., Alou M.T., Daillère R., Fluckiger A., Messaoudene M., Rauber C., Roberti M.P. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 12.Gopalakrishnan V., Spencer C.N., Nezi L., Reuben A., Andrews M., Karpinets T., Prieto P., Vicente D., Hoffman K., Wei S.C. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matson V., Fessler J., Bao R., Chongsuwat T., Zha Y., Alegre M.-L., Luke J.J., Gajewski T.F. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W.N., Deng Y., Chu Q., Zhang P. Gut microbiome and cancer immunotherapy. Cancer Lett. 2019;447:41–47. doi: 10.1016/j.canlet.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Rivalland G., Scott A.M., John T. Standard of care in immunotherapy trials: Challenges and considerations. Hum. Vaccin. Immunother. 2017;13:2164–2178. doi: 10.1080/21645515.2016.1277845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naimi A., Mohammed R.N., Raji A., Chupradit S., Yumashev A.V., Suksatan W., Shalaby M.N., Thangavelu L., Kamrava S., Shomali N., et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun. Signal. 2022;20:44. doi: 10.1186/s12964-022-00854-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham N., Lapointe R., Lerouge S. Biomaterials for enhanced immunotherapy. APL Bioeng. 2022;6:041502. doi: 10.1063/5.0125692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wojtukiewicz M.Z., Rek M.M., Karpowicz K., Górska M., Polityńska B., Wojtukiewicz A.M., Moniuszko M., Radziwon P., Tucker S.C., Honn K.V. Inhibitors of immune checkpoints-PD-1, PD-L1, CTLA-4-new opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer Metastasis Rev. 2021;40:949–982. doi: 10.1007/s10555-021-09976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munir S., Lundsager M.T., Jørgensen M.A., Hansen M., Petersen T.H., Bonefeld C.M., Friese C., Met Ö., Straten P.T., Andersen M.H. Inflammation induced PD-L1-specific T cells. Cell Stress. 2019;3:319–327. doi: 10.15698/cst2019.10.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alsaab H.O., Sau S., Alzhrani R., Tatiparti K., Bhise K., Kashaw S.K., Iyer A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017;8:561. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoo S.Y., Badrinath N., Jeong S.N., Woo H.Y., Heo J. Overcoming Tumor Resistance to Oncolyticvaccinia Virus with Anti-PD-1-Based Combination Therapy by Inducing Antitumor Immunity in the Tumor Microenvironment. Vaccines. 2020;8:321. doi: 10.3390/vaccines8020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin S., Xu L., Yi M., Yu S., Wu K., Luo S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer. 2019;18:155. doi: 10.1186/s12943-019-1091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seidel J.A., Otsuka A., Kabashima K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018;8:86. doi: 10.3389/fonc.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vafaei S., Zekiy A.O., Khanamir R.A., Zaman B.A., Ghayourvahdat A., Azimizonuzi H., Zamani M. Combination therapy with immune checkpoint inhibitors (ICIs); a new frontier. Cancer Cell Int. 2022;22:2. doi: 10.1186/s12935-021-02407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bajwa R., Cheema A., Khan T., Amirpour A., Paul A., Chaughtai S., Patel S., Patel T., Bramson J., Gupta V., et al. Adverse Effects of Immune Checkpoint Inhibitors (Programmed Death-1 Inhibitors and Cytotoxic T-Lymphocyte-Associated Protein-4 Inhibitors): Results of a Retrospective Study. J. Clin. Med. Res. 2019;11:225–236. doi: 10.14740/jocmr3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franzin R., Netti G.S., Spadaccino F., Porta C., Gesualdo L., Stallone G., Castellano G., Ranieri E. The Use of Immune Checkpoint Inhibitors in Oncology and the Occurrence of AKI: Where Do We Stand? Front. Immunol. 2020;11:574271. doi: 10.3389/fimmu.2020.574271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang X.Y., Shi A.P., Xiong Y.L., Zheng K.F., Liu Y.J., Shi X.G., Jiang T., Zhao J.B. Clinical Research on the Mechanisms Underlying Immune Checkpoints and Tumor Metastasis. Front. Oncol. 2021;11:693321. doi: 10.3389/fonc.2021.693321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conlon K.C., Miljkovic M.D., Waldmann T.A. Cytokines in the Treatment of Cancer. J. Interferon Cytokine Res. 2019;39:6–21. doi: 10.1089/jir.2018.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jorgovanovic D., Song M., Wang L., Zhang Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020;8:49. doi: 10.1186/s40364-020-00228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Den Eeckhout B., Tavernier J., Gerlo S. Interleukin-1 as Innate Mediator of T Cell Immunity. Front. Immunol. 2021;11:621931. doi: 10.3389/fimmu.2020.621931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mashima H., Zhang R., Kobayashi T., Hagiya Y., Tsukamoto H., Liu T., Iwama T., Yamamoto M., Lin C., Nakatsuka R., et al. Generation of GM-CSF-producing antigen-presenting cells that induce a cytotoxic T cell-mediated antitumor response. Oncoimmunology. 2020;9:1814620. doi: 10.1080/2162402X.2020.1814620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanborn R.E., Schneiders F.L., Senan S., Gadgeel S.M. Beyond Checkpoint Inhibitors: Enhancing Antitumor Immune Response in Lung Cancer. Am. Soc. Clin. Oncol. Educ. Book. 2022;41:673–686. doi: 10.1200/EDBK_350967. [DOI] [PubMed] [Google Scholar]
- 33.Sadeghi Najafabadi S.A., Bolhassani A., Aghasadeghi M.R. Tumor cell-based vaccine: An effective strategy for eradication of cancer cells. Immunotherapy. 2022;14:639–654. doi: 10.2217/imt-2022-0036. [DOI] [PubMed] [Google Scholar]
- 34.Palucka K., Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He Q., Gao H., Tan D., Zhang H., Wang J.-z. mRNA cancer vaccines: Advances, trends and challenges. Acta Pharm. Sin. B. 2022;12:2969–2989. doi: 10.1016/j.apsb.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu C., Zhou L., Mi Q.-S., Jiang A. DC-Based Vaccines for Cancer Immunotherapy. Vaccines. 2020;8:706. doi: 10.3390/vaccines8040706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li D., Li X., Zhou W.-L., Huang Y., Liang X., Jiang L., Yang X., Sun J., Li Z., Han W.-D., et al. Genetically engineered T cells for cancer immunotherapy. Signal Transduct. Target. Ther. 2019;4:35. doi: 10.1038/s41392-019-0070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hammerstrom A.E., Cauley D.H., Atkinson B.J., Sharma P. Cancer immunotherapy: Sipuleucel-T and beyond. Pharmacotherapy. 2011;31:813–828. doi: 10.1592/phco.31.8.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saxena M., Balan S., Roudko V., Bhardwaj N. Towards superior dendritic-cell vaccines for cancer therapy. Nat. Biomed. Eng. 2018;2:341–346. doi: 10.1038/s41551-018-0250-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbier A.J., Jiang A.Y., Zhang P., Wooster R., Anderson D.G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022;40:840–854. doi: 10.1038/s41587-022-01294-2. [DOI] [PubMed] [Google Scholar]
- 41.Wadhwa A., Aljabbari A., Lokras A., Foged C., Thakur A. Opportunities and Challenges in the Delivery of mRNA-based Vaccines. Pharmaceutics. 2020;12:102. doi: 10.3390/pharmaceutics12020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng M., Mo Y., Wang Y., Wu P., Zhang Y., Xiong F., Guo C., Wu X., Li Y., Li X., et al. Neoantigen vaccine: An emerging tumor immunotherapy. Mol. Cancer. 2019;18:128. doi: 10.1186/s12943-019-1055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Truong C.S., Yoo S.Y. Oncolytic Vaccinia Virus in Lung Cancer Vaccines. Vaccines. 2022;10:240. doi: 10.3390/vaccines10020240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mardis E.R. Neoantigens and genome instability: Impact on immunogenomic phenotypes and immunotherapy response. Genome Med. 2019;11:71. doi: 10.1186/s13073-019-0684-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salomon R., Dahan R. Next Generation CD40 Agonistic Antibodies for Cancer Immunotherapy. Front. Immunol. 2022;13:940674. doi: 10.3389/fimmu.2022.940674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vonderheide R.H. CD40 Agonist Antibodies in Cancer Immunotherapy. Annu. Rev. Med. 2020;71:47–58. doi: 10.1146/annurev-med-062518-045435. [DOI] [PubMed] [Google Scholar]
- 47.van Kooten C., Banchereau J. CD40-CD40 ligand. J. Leukoc. Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- 48.Chester C., Sanmamed M.F., Wang J., Melero I. Immunotherapy targeting 4-1BB: Mechanistic rationale, clinical results, and future strategies. Blood. 2018;131:49–57. doi: 10.1182/blood-2017-06-741041. [DOI] [PubMed] [Google Scholar]
- 49.Segal N.H., Logan T.F., Hodi F.S., McDermott D., Melero I., Hamid O., Schmidt H., Robert C., Chiarion-Sileni V., Ascierto P.A., et al. Results from an Integrated Safety Analysis of Urelumab, an Agonist Anti-CD137 Monoclonal Antibody. Clin. Cancer Res. 2017;23:1929–1936. doi: 10.1158/1078-0432.CCR-16-1272. [DOI] [PubMed] [Google Scholar]
- 50.Tolcher A.W., Sznol M., Hu-Lieskovan S., Papadopoulos K.P., Patnaik A., Rasco D.W., Di Gravio D., Huang B., Gambhire D., Chen Y., et al. Phase Ib Study of Utomilumab (PF-05082566), a 4-1BB/CD137 Agonist, in Combination with Pembrolizumab (MK-3475) in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017;23:5349–5357. doi: 10.1158/1078-0432.CCR-17-1243. [DOI] [PubMed] [Google Scholar]
- 51.Buchan S.L., Rogel A., Al-Shamkhani A. The immunobiology of CD27 and OX40 and their potential as targets for cancer immunotherapy. Blood. 2018;131:39–48. doi: 10.1182/blood-2017-07-741025. [DOI] [PubMed] [Google Scholar]
- 52.Zhao L., Cao Y.J. Engineered T Cell Therapy for Cancer in the Clinic. Front. Immunol. 2019;10:2250. doi: 10.3389/fimmu.2019.02250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jogalekar M.P., Rajendran R.L., Khan F., Dmello C., Gangadaran P., Ahn B.C. CAR T-Cell-Based gene therapy for cancers: New perspectives, challenges, and clinical developments. Front. Immunol. 2022;13:925985. doi: 10.3389/fimmu.2022.925985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scholler J., Brady T.L., Binder-Scholl G., Hwang W.T., Plesa G., Hege K.M., Vogel A.N., Kalos M., Riley J.L., Deeks S.G., et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med. 2012;4:132ra153. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abou-El-Enein M., Elsallab M., Feldman S.A., Fesnak A.D., Heslop H.E., Marks P., Till B.G., Bauer G., Savoldo B. Scalable Manufacturing of CAR T cells for Cancer Immunotherapy. Blood Cancer Discov. 2021;2:408–422. doi: 10.1158/2643-3230.BCD-21-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marofi F., Motavalli R., Safonov V.A., Thangavelu L., Yumashev A.V., Alexander M., Shomali N., Chartrand M.S., Pathak Y., Jarahian M., et al. CAR T cells in solid tumors: Challenges and opportunities. Stem Cell Res. Ther. 2021;12:81. doi: 10.1186/s13287-020-02128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Al-Haideri M., Tondok S.B., Safa S.H., maleki A.H., Rostami S., Jalil A.T., Al-Gazally M.E., Alsaikhan F., Rizaev J.A., Mohammad T.A.M., et al. CAR-T cell combination therapy: The next revolution in cancer treatment. Cancer Cell Int. 2022;22:365. doi: 10.1186/s12935-022-02778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sengsayadeth S., Savani B.N., Oluwole O., Dholaria B. Overview of approved CAR-T therapies, ongoing clinical trials, and its impact on clinical practice. EJHaem. 2022;3:6–10. doi: 10.1002/jha2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ping Y., Liu C., Zhang Y. T-cell receptor-engineered T cells for cancer treatment: Current status and future directions. Protein Cell. 2018;9:254–266. doi: 10.1007/s13238-016-0367-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharpe M., Mount N. Genetically modified T cells in cancer therapy: Opportunities and challenges. Dis. Model. Mech. 2015;8:337–350. doi: 10.1242/dmm.018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santos Apolonio J., Lima de Souza Gonçalves V., Cordeiro Santos M.L., Silva Luz M., Silva Souza J.V., Rocha Pinheiro S.L., de Souza W.R., Sande Loureiro M., de Melo F.F. Oncolytic virus therapy in cancer: A current review. World J. Virol. 2021;10:229–255. doi: 10.5501/wjv.v10.i5.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoo S.Y., Badrinath N., Woo H.Y., Heo J. Oncolytic Virus-Based Immunotherapies for Hepatocellular Carcinoma. Mediat. Inflamm. 2017;2017:5198798. doi: 10.1155/2017/5198798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Singh P.K., Doley J., Kumar G.R., Sahoo A.P., Tiwari A.K. Oncolytic viruses & their specific targeting to tumour cells. Indian J. Med. Res. 2012;136:571–584. [PMC free article] [PubMed] [Google Scholar]
- 64.Badrinath N., Heo J., Yoo S.Y. Viruses as nanomedicine for cancer. Int. J. Nanomed. 2016;11:4835–4847. doi: 10.2147/ijn.S116447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jeong S.N., Yoo S.Y. Novel Oncolytic Virus Armed with Cancer Suicide Gene and Normal Vasculogenic Gene for Improved Anti-Tumor Activity. Cancers. 2020;12:1070. doi: 10.3390/cancers12051070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang L., Gu X., Yu J., Ge S., Fan X. Oncolytic Virotherapy: From Bench to Bedside. Front. Cell Dev. Biol. 2021;9:790150. doi: 10.3389/fcell.2021.790150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramesh N., Ge Y., Ennist D.L., Zhu M., Mina M., Ganesh S., Reddy P.S., Yu D.C. CG0070, a conditionally replicating granulocyte macrophage colony-stimulating factor--armed oncolytic adenovirus for the treatment of bladder cancer. Clin. Cancer Res. 2006;12:305–313. doi: 10.1158/1078-0432.CCR-05-1059. [DOI] [PubMed] [Google Scholar]
- 68.Ruano D., López-Martín J.A., Moreno L., Lassaletta Á., Bautista F., Andión M., Hernández C., González-Murillo Á., Melen G., Alemany R., et al. First-in-Human, First-in-Child Trial of Autologous MSCs Carrying the Oncolytic Virus Icovir-5 in Patients with Advanced Tumors. Mol. Ther. 2020;28:1033–1042. doi: 10.1016/j.ymthe.2020.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim J.H., Oh J.Y., Park B.H., Lee D.E., Kim J.S., Park H.E., Roh M.S., Je J.E., Yoon J.H., Thorne S.H., et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol. Ther. 2006;14:361–370. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 70.Yoo S.Y., Bang S.Y., Jeong S.N., Kang D.H., Heo J. A cancer-favoring oncolytic vaccinia virus shows enhanced suppression of stem-cell like colon cancer. Oncotarget. 2016;7:16479–16489. doi: 10.18632/oncotarget.7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yoo S.Y., Jeong S.N., Kang D.H., Heo J. Evolutionary cancer-favoring engineered vaccinia virus for metastatic hepatocellular carcinoma. Oncotarget. 2017;8:71489–71499. doi: 10.18632/oncotarget.17288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Poh A. First Oncolytic Viral Therapy for Melanoma. Cancer Discov. 2016;6:6. doi: 10.1158/2159-8290.Cd-nb2015-158. [DOI] [PubMed] [Google Scholar]
- 73.Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021;20:101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ailia M.J., Yoo S.Y. In Vivo Oncolytic Virotherapy in Murine Models of Hepatocellular Carcinoma: A Systematic Review. Vaccines. 2022;10:1541. doi: 10.3390/vaccines10091541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shrestha K.R., Lee D.H., Chung W., Lee S.W., Lee B.Y., Yoo S.Y. Biomimetic virus-based soft niche for ischemic diseases. Biomaterials. 2022;288:121747. doi: 10.1016/j.biomaterials.2022.121747. [DOI] [PubMed] [Google Scholar]
- 76.Melero I., Castanon E., Alvarez M., Champiat S., Marabelle A. Intratumoural administration and tumour tissue targeting of cancer immunotherapies. Nat. Rev. Clin. Oncol. 2021;18:558–576. doi: 10.1038/s41571-021-00507-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Crittenden M.R., Thanarajasingam U., Vile R.G., Gough M.J. Intratumoral immunotherapy: Using the tumour against itself. Immunology. 2005;114:11–22. doi: 10.1111/j.1365-2567.2004.02001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rabinow B.E. Nanosuspensions in drug delivery. Nat. Rev. Drug Discov. 2004;3:785–796. doi: 10.1038/nrd1494. [DOI] [PubMed] [Google Scholar]
- 79.Porter C.J., Trevaskis N.L., Charman W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007;6:231–248. doi: 10.1038/nrd2197. [DOI] [PubMed] [Google Scholar]
- 80.Chaudhary S., Garg T., Murthy R., Rath G., Goyal A.K. Recent approaches of lipid-based delivery system for lymphatic targeting via oral route. J. Drug Target. 2014;22:871–882. doi: 10.3109/1061186X.2014.950664. [DOI] [PubMed] [Google Scholar]
- 81.Kratz F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release. 2008;132:171–183. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 82.Vaz J., Ansari D., Sasor A., Andersson R. SPARC: A potential prognostic and therapeutic target in pancreatic cancer. Pancreas. 2015;44:1024. doi: 10.1097/MPA.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cortes J.E., Goldberg S.L., Feldman E.J., Rizzeri D.A., Hogge D.E., Larson M., Pigneux A., Recher C., Schiller G., Warzocha K. Phase II, multicenter, randomized trial of CPX-351 (cytarabine: Daunorubicin) liposome injection versus intensive salvage therapy in adults with first relapse AML. Cancer. 2015;121:234–242. doi: 10.1002/cncr.28974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sartor O. Eligard: Leuprolide acetate in a novel sustained-release delivery system. Urology. 2003;61:25–31. doi: 10.1016/S0090-4295(02)02396-8. [DOI] [PubMed] [Google Scholar]
- 85.Podust V.N., Balan S., Sim B.-C., Coyle M.P., Ernst U., Peters R.T., Schellenberger V. Extension of in vivo half-life of biologically active molecules by XTEN protein polymers. J. Control. Release. 2016;240:52–66. doi: 10.1016/j.jconrel.2015.10.038. [DOI] [PubMed] [Google Scholar]
- 86.Lee S.H., Kim B.H., Park C.G., Lee C., Lim B.Y., Choy Y.B. Implantable small device enabled with magnetic actuation for on-demand and pulsatile drug delivery. J. Control. Release. 2018;286:224–230. doi: 10.1016/j.jconrel.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 87.Roth A., Rohrbach F., Weth R., Frisch B., Schuber F., Wels W.S. Induction of effective and antigen-specific antitumour immunity by a liposomal ErbB2/HER2 peptide-based vaccination construct. Br. J. Cancer. 2005;92:1421–1429. doi: 10.1038/sj.bjc.6602526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yuba E., Harada A., Sakanishi Y., Watarai S., Kono K. A liposome-based antigen delivery system using pH-sensitive fusogenic polymers for cancer immunotherapy. Biomaterials. 2013;34:3042–3052. doi: 10.1016/j.biomaterials.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 89.Zheng Y., Stephan M.T., Gai S.A., Abraham W., Shearer A., Irvine D.J. In vivo targeting of adoptively transferred T-cells with antibody- and cytokine-conjugated liposomes. J. Control. Release. 2013;172:426–435. doi: 10.1016/j.jconrel.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aoyama K., Kuroda S., Morihiro T., Kanaya N., Kubota T., Kakiuchi Y., Kikuchi S., Nishizaki M., Kagawa S., Tazawa H., et al. Liposome-encapsulated plasmid DNA of telomerase-specific oncolytic adenovirus with stealth effect on the immune system. Sci. Rep. 2017;7:14177. doi: 10.1038/s41598-017-14717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu J., Liu X., Han Y., Zhang J., Liu D., Ma G., Li C., Liu L., Kong D. Nanovaccine Incorporated with Hydroxychloroquine Enhances Antigen Cross-Presentation and Promotes Antitumor Immune Responses. ACS Appl. Mater. Interfaces. 2018;10:30983–30993. doi: 10.1021/acsami.8b09348. [DOI] [PubMed] [Google Scholar]
- 92.Reda M., Ngamcherdtrakul W., Nelson M.A., Siriwon N., Wang R., Zaidan H.Y., Bejan D.S., Reda S., Hoang N.H., Crumrine N.A., et al. Development of a nanoparticle-based immunotherapy targeting PD-L1 and PLK1 for lung cancer treatment. Nat. Commun. 2022;13:4261. doi: 10.1038/s41467-022-31926-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu Y., Li J., Song B., Ma Z., Zhang Y., Sun H., Wei X., Bai Y., Lu X., Zhang P., et al. Polymeric PD-L1 blockade nanoparticles for cancer photothermal-immunotherapy. Biomaterials. 2022;280:121312. doi: 10.1016/j.biomaterials.2021.121312. [DOI] [PubMed] [Google Scholar]
- 94.Huang K.-W., Hsu F.-F., Qiu J.T., Qiu J.T., Chern G.-J., Lee Y.-A., Chang C.-C., Huang Y.-T., Sung Y.-C., Chiang C.-C., et al. Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer. Sci. Adv. 2020;6:eaax5032. doi: 10.1126/sciadv.aax5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roy D.G., Bell J.C., Bourgeois-Daigneault M.C. Magnetic targeting of oncolytic VSV-based therapies improves infection of tumor cells in the presence of virus-specific neutralizing antibodies in vitro. Biochem. Biophys. Res. Commun. 2020;526:641–646. doi: 10.1016/j.bbrc.2020.03.135. [DOI] [PubMed] [Google Scholar]
- 96.Tresilwised N., Pithayanukul P., Mykhaylyk O., Holm P.S., Holzmüller R., Anton M., Thalhammer S., Adigüzel D., Döblinger M., Plank C. Boosting Oncolytic Adenovirus Potency with Magnetic Nanoparticles and Magnetic Force. Mol. Pharm. 2010;7:1069–1089. doi: 10.1021/mp100123t. [DOI] [PubMed] [Google Scholar]
- 97.Yin Y., Li X., Ma H., Zhang J., Yu D., Zhao R., Yu S., Nie G., Wang H. In Situ Transforming RNA Nanovaccines from Polyethylenimine Functionalized Graphene Oxide Hydrogel for Durable Cancer Immunotherapy. Nano Lett. 2021;21:2224–2231. doi: 10.1021/acs.nanolett.0c05039. [DOI] [PubMed] [Google Scholar]
- 98.Wang C., Sun W., Wright G., Wang A.Z., Gu Z. Inflammation-Triggered Cancer Immunotherapy by Programmed Delivery of CpG and Anti-PD1 Antibody. Adv. Mater. 2016;28:8912–8920. doi: 10.1002/adma.201506312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu S., Jiang Q., Zhao X., Zhao R., Wang Y., Wang Y., Liu J., Shang Y., Zhao S., Wu T., et al. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat. Mater. 2021;20:421–430. doi: 10.1038/s41563-020-0793-6. [DOI] [PubMed] [Google Scholar]
- 100.Ohno S., Takanashi M., Sudo K., Ueda S., Ishikawa A., Matsuyama N., Fujita K., Mizutani T., Ohgi T., Ochiya T., et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 2013;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kooijmans S.A., Aleza C.G., Roffler S.R., van Solinge W.W., Vader P., Schiffelers R.M. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J. Extracell. Vesicles. 2016;5:31053. doi: 10.3402/jev.v5.31053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang K., Zhang Y., Zhang H., Xu P., Liu J., Ma J., Lv M., Li D., Katirai F., Shen G.-X., et al. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat. Commun. 2012;3:1282. doi: 10.1038/ncomms2282. [DOI] [PubMed] [Google Scholar]
- 103.Ni Q., Zhang F., Liu Y., Wang Z., Yu G., Liang B., Niu G., Su T., Zhu G., Lu G., et al. A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer. Sci. Adv. 2020;6:eaaw6071. doi: 10.1126/sciadv.aaw6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y., Shen T., Zhou S., Wang W., Lin S., Zhu G. pH-Responsive STING-Activating DNA Nanovaccines for Cancer Immunotherapy. Adv. Ther. 2020;3:2000083. doi: 10.1002/adtp.202000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Y., Chen X., Tian B., Liu J., Yang L., Zeng L., Chen T., Hong A., Wang X. Nucleolin-targeted Extracellular Vesicles as a Versatile Platform for Biologics Delivery to Breast Cancer. Theranostics. 2017;7:1360–1372. doi: 10.7150/thno.16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yuan Z., Kolluri K.K., Gowers K.H., Janes S.M. TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. J. Extracell. Vesicles. 2017;6:1265291. doi: 10.1080/20013078.2017.1265291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ma J., Zhang Y., Tang K., Zhang H., Yin X., Li Y., Xu P., Sun Y., Ma R., Ji T., et al. Reversing drug resistance of soft tumor-repopulating cells by tumor cell-derived chemotherapeutic microparticles. Cell Res. 2016;26:713–727. doi: 10.1038/cr.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Morishita M., Takahashi Y., Matsumoto A., Nishikawa M., Takakura Y. Exosome-based tumor antigens-adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials. 2016;111:55–65. doi: 10.1016/j.biomaterials.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 109.Liang Q., Bie N., Yong T., Tang K., Shi X., Wei Z., Jia H., Zhang X., Zhao H., Huang W., et al. The softness of tumour-cell-derived microparticles regulates their drug-delivery efficiency. Nat. Biomed. Eng. 2019;3:729–740. doi: 10.1038/s41551-019-0405-4. [DOI] [PubMed] [Google Scholar]
- 110.Phuengkham H., Song C., Um S.H., Lim Y.T. Implantable Synthetic Immune Niche for Spatiotemporal Modulation of Tumor-Derived Immunosuppression and Systemic Antitumor Immunity: Postoperative Immunotherapy. Adv. Mater. 2018;30:e1706719. doi: 10.1002/adma.201706719. [DOI] [PubMed] [Google Scholar]
- 111.Ali O.A., Huebsch N., Cao L., Dranoff G., Mooney D.J. Infection-mimicking materials to program dendritic cells in situ. Nat. Mater. 2009;8:151–158. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ahn Y.H., Ren L., Kim S.M., Seo S.H., Jung C.R., Kim D.S., Noh J.Y., Lee S.Y., Lee H., Cho M.Y., et al. A three-dimensional hyaluronic acid-based niche enhances the therapeutic efficacy of human natural killer cell-based cancer immunotherapy. Biomaterials. 2020;247:119960. doi: 10.1016/j.biomaterials.2020.119960. [DOI] [PubMed] [Google Scholar]
- 113.Li Y., Fang M., Zhang J., Wang J., Song Y., Shi J., Li W., Wu G., Ren J., Wang Z., et al. Hydrogel dual delivered celecoxib and anti-PD-1 synergistically improve antitumor immunity. Oncoimmunology. 2016;5:e1074374. doi: 10.1080/2162402X.2015.1074374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Song H., Huang P., Niu J., Shi G., Zhang C., Kong D., Wang W. Injectable polypeptide hydrogel for dual-delivery of antigen and TLR3 agonist to modulate dendritic cells in vivo and enhance potent cytotoxic T-lymphocyte response against melanoma. Biomaterials. 2018;159:119–129. doi: 10.1016/j.biomaterials.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 115.Wang C., Wang J., Zhang X., Yu S., Wen D., Hu Q., Ye Y., Bomba H., Hu X., Liu Z., et al. In situ formed reactive oxygen species-responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Sci. Transl. Med. 2018;10:eaan3682. doi: 10.1126/scitranslmed.aan3682. [DOI] [PubMed] [Google Scholar]
- 116.Xie X., Wang H., Williams G.R., Yang Y., Zheng Y., Wu J., Zhu L.M. Erythrocyte Membrane Cloaked Curcumin-Loaded Nanoparticles for Enhanced Chemotherapy. Pharmaceutics. 2019;11:429. doi: 10.3390/pharmaceutics11090429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang L., Wang Z., Zhang Y., Cao F., Dong K., Ren J., Qu X. Erythrocyte Membrane Cloaked Metal-Organic Framework Nanoparticle as Biomimetic Nanoreactor for Starvation-Activated Colon Cancer Therapy. ACS Nano. 2018;12:10201–10211. doi: 10.1021/acsnano.8b05200. [DOI] [PubMed] [Google Scholar]
- 118.Luk B.T., Fang R.H., Hu C.-M.J., Copp J.A., Thamphiwatana S., Dehaini D., Gao W., Zhang K., Li S., Zhang L. Safe and Immunocompatible Nanocarriers Cloaked in RBC Membranes for Drug Delivery to Treat Solid Tumors. Theranostics. 2016;6:1004–1011. doi: 10.7150/thno.14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang L., Li R., Chen H., Wei J., Qian H., Su S., Shao J., Wang L., Qian X., Liu B. Human cytotoxic T-lymphocyte membrane-camouflaged nanoparticles combined with low-dose irradiation: A new approach to enhance drug targeting in gastric cancer. Int. J. Nanomed. 2017;12:2129–2142. doi: 10.2147/IJN.S126016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deng G., Sun Z., Li S., Peng X., Li W., Zhou L., Ma Y., Gong P., Cai L. Cell-membrane immunotherapy based on natural killer cell membrane coated nanoparticles for the effective inhibition of primary and abscopal tumor growth. ACS Nano. 2018;12:12096–12108. doi: 10.1021/acsnano.8b05292. [DOI] [PubMed] [Google Scholar]
- 121.Sridhar P., Petrocca F. Regional Delivery of Chimeric Antigen Receptor (CAR) T-Cells for Cancer Therapy. Cancers. 2017;9:92. doi: 10.3390/cancers9070092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cheng Z., Li M., Dey R., Chen Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021;14:85. doi: 10.1186/s13045-021-01096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Waheed S., Li Z., Zhang F., Chiarini A., Armato U., Wu J. Engineering nano-drug biointerface to overcome biological barriers toward precision drug delivery. J. Nanobiotechnology. 2022;20:395. doi: 10.1186/s12951-022-01605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou Y., Chen X., Cao J., Gao H. Overcoming the biological barriers in the tumor microenvironment for improving drug delivery and efficacy. J. Mater. Chem. B. 2020;8:6765–6781. doi: 10.1039/D0TB00649A. [DOI] [PubMed] [Google Scholar]
- 125.Hosseini M., Haji-Fatahaliha M., Jadidi-Niaragh F., Majidi J., Yousefi M. The use of nanoparticles as a promising therapeutic approach in cancer immunotherapy. Artif. Cells Nanomed. Biotechnol. 2016;44:1051–1061. doi: 10.3109/21691401.2014.998830. [DOI] [PubMed] [Google Scholar]
- 126.Yao Y., Zhou Y., Liu L., Xu Y., Chen Q., Wang Y., Wu S., Deng Y., Zhang J., Shao A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020;7:193. doi: 10.3389/fmolb.2020.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cheng Y.-H., He C., Riviere J.E., Monteiro-Riviere N.A., Lin Z. Meta-Analysis of Nanoparticle Delivery to Tumors Using a Physiologically Based Pharmacokinetic Modeling and Simulation Approach. ACS Nano. 2020;14:3075–3095. doi: 10.1021/acsnano.9b08142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pudlarz A., Szemraj J. Nanoparticles as Carriers of Proteins, Peptides and Other Therapeutic Molecules. Open Life Sci. 2018;13:285–298. doi: 10.1515/biol-2018-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shinde V.R., Revi N., Murugappan S., Singh S.P., Rengan A.K. Enhanced permeability and retention effect: A key facilitator for solid tumor targeting by nanoparticles. Photodiagnosis Photodyn. Ther. 2022;39:102915. doi: 10.1016/j.pdpdt.2022.102915. [DOI] [PubMed] [Google Scholar]
- 130.Zhang J., Fan B., Cao G., Huang W., Jia F., Nie G., Wang H. Direct Presentation of Tumor-Associated Antigens to Induce Adaptive Immunity by Personalized Dendritic Cell-Mimicking Nanovaccines. Adv. Mater. 2022;34:2205950. doi: 10.1002/adma.202205950. [DOI] [PubMed] [Google Scholar]
- 131.Zhen Z., Tang W., Wang M., Zhou S., Wang H., Wu Z., Hao Z., Li Z., Liu L., Xie J. Protein Nanocage Mediated Fibroblast-Activation Protein Targeted Photoimmunotherapy To Enhance Cytotoxic T Cell Infiltration and Tumor Control. Nano Lett. 2017;17:862–869. doi: 10.1021/acs.nanolett.6b04150. [DOI] [PubMed] [Google Scholar]
- 132.Wu Y.-P., Yang J., Gao H.-Y., Shen Y., Jiang L., Zhou C., Li Y.-F., He R.-R., Liu M. Folate-Conjugated Halloysite Nanotubes, an Efficient Drug Carrier, Deliver Doxorubicin for Targeted Therapy of Breast Cancer. ACS Appl. Nano Mater. 2018;1:595–608. doi: 10.1021/acsanm.7b00087. [DOI] [Google Scholar]
- 133.Wang H., Li Z., Lu S., Li C., Zhao W., Zhao Y., Yu S., Wang T., Sun T. Nano micelles of cellulose-graft-poly (l-lactic acid) anchored with epithelial cell adhesion antibody for enhanced drug loading and anti-tumor effect. Mater. Today Commun. 2020;22:100764. doi: 10.1016/j.mtcomm.2019.100764. [DOI] [Google Scholar]
- 134.Chiang C.-S., Lin Y.-J., Lee R., Lai Y.-H., Cheng H.-W., Hsieh C.-H., Shyu W.-C., Chen S.-Y. Combination of fucoidan-based magnetic nanoparticles and immunomodulators enhances tumour-localized immunotherapy. Nat. Nanotechnol. 2018;13:746–754. doi: 10.1038/s41565-018-0146-7. [DOI] [PubMed] [Google Scholar]
- 135.Badrinath N., Jeong Y.I., Woo H.Y., Bang S.Y., Kim C., Heo J., Kang D.H., Yoo S.Y. Local delivery of a cancer-favoring oncolytic vaccinia virus via poly (lactic-co-glycolic acid) nanofiber for theranostic purposes. Int. J. Pharm. 2018;552:437–442. doi: 10.1016/j.ijpharm.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 136.Mohapatra A., Sathiyamoorthy P., Park I.K. Metallic Nanoparticle-Mediated Immune Cell Regulation and Advanced Cancer Immunotherapy. Pharmaceutics. 2021;13:1867. doi: 10.3390/pharmaceutics13111867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fang X., Lan H., Jin K., Gong D., Qian J. Nanovaccines for Cancer Prevention and Immunotherapy: An Update Review. Cancers. 2022;14:3842. doi: 10.3390/cancers14163842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Paston S.J., Brentville V.A., Symonds P., Durrant L.G. Cancer Vaccines, Adjuvants, and Delivery Systems. Front. Immunol. 2021;12:627932. doi: 10.3389/fimmu.2021.627932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jin L., Yang D., Song Y., Li D., Xu W., Zhu Y., Xu C.F., Lu Y., Yang X. In Situ Programming of Nanovaccines for Lymph Node-Targeted Delivery and Cancer Immunotherapy. ACS Nano. 2022;16:15226–15236. doi: 10.1021/acsnano.2c06560. [DOI] [PubMed] [Google Scholar]
- 140.Li X., Zhang Y., Wu X., Chen J., Yang M., Ma F., Shi L. In Situ Antigen-Capturing Nanochaperone Toward Personalized Nanovaccine for Cancer Immunotherapy. Small. 2022;18:2203100. doi: 10.1002/smll.202203100. [DOI] [PubMed] [Google Scholar]
- 141.Xu J., Lv J., Zhuang Q., Yang Z., Cao Z., Xu L., Pei P., Wang C., Wu H., Dong Z., et al. A general strategy towards personalized nanovaccines based on fluoropolymers for post-surgical cancer immunotherapy. Nat. Nanotechnol. 2020;15:1043–1052. doi: 10.1038/s41565-020-00781-4. [DOI] [PubMed] [Google Scholar]
- 142.Luo Z., He T., Liu P., Yi Z., Zhu S., Liang X., Kang E., Gong C., Liu X. Self-Adjuvanted Molecular Activator (SeaMac) Nanovaccines Promote Cancer Immunotherapy. Adv. Healthc. Mater. 2021;10:2002080. doi: 10.1002/adhm.202002080. [DOI] [PubMed] [Google Scholar]
- 143.Wang J., Wu X., Shen P., Wang J., Shen Y., Shen Y., Webster T.J., Deng J. Applications of Inorganic Nanomaterials in Photothermal Therapy Based on Combinational Cancer Treatment. Int. J. Nanomed. 2020;15:1903–1914. doi: 10.2147/IJN.S239751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Montaseri H., Kruger C.A., Abrahamse H. Review: Organic nanoparticle based active targeting for photodynamic therapy treatment of breast cancer cells. Oncotarget. 2020;11:2120–2136. doi: 10.18632/oncotarget.27596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim J., Chun S.H., Amornkitbamrung L., Song C., Yuk J.S., Ahn S.Y., Kim B.W., Lim Y.T., Oh B.K., Um S.H. Gold nanoparticle clusters for the investigation of therapeutic efficiency against prostate cancer under near-infrared irradiation. Nano Converg. 2020;7:5. doi: 10.1186/s40580-019-0216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lu Z., Bai S., Jiang Y., Wu S., Xu D., Zhang J., Peng X., Zhang H., Shi Y., Liu G. Amplifying Dendritic Cell Activation by Bioinspired Nanometal Organic Frameworks for Synergistic Sonoimmunotherapy. Small. 2022;18:e2203952. doi: 10.1002/smll.202203952. [DOI] [PubMed] [Google Scholar]
- 147.Alili L., Sack M., Karakoti A.S., Teuber S., Puschmann K., Hirst S.M., Reilly C.M., Zanger K., Stahl W., Das S. Combined cytotoxic and anti-invasive properties of redox-active nanoparticles in tumor–stroma interactions. Biomaterials. 2011;32:2918–2929. doi: 10.1016/j.biomaterials.2010.12.056. [DOI] [PubMed] [Google Scholar]
- 148.Mardhian D.F., Storm G., Bansal R., Prakash J. Nano-targeted relaxin impairs fibrosis and tumor growth in pancreatic cancer and improves the efficacy of gemcitabine in vivo. J. Control. Release. 2018;290:1–10. doi: 10.1016/j.jconrel.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 149.Hu C., Liu X., Ran W., Meng J., Zhai Y., Zhang P., Yin Q., Yu H., Zhang Z., Li Y. Regulating cancer associated fibroblasts with losartan-loaded injectable peptide hydrogel to potentiate chemotherapy in inhibiting growth and lung metastasis of triple negative breast cancer. Biomaterials. 2017;144:60–72. doi: 10.1016/j.biomaterials.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 150.Chen X., Zhou W., Liang C., Shi S., Yu X., Chen Q., Sun T., Lu Y., Zhang Y., Guo Q. Codelivery nanosystem targeting the deep microenvironment of pancreatic cancer. Nano Lett. 2019;19:3527–3534. doi: 10.1021/acs.nanolett.9b00374. [DOI] [PubMed] [Google Scholar]
- 151.Zanganeh S., Hutter G., Spitler R., Lenkov O., Mahmoudi M., Shaw A., Pajarinen J.S., Nejadnik H., Goodman S., Moseley M. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016;11:986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sang Y., Deng Q., Cao F., Liu Z., You Y., Liu H., Ren J., Qu X. Remodeling Macrophages by an Iron Nanotrap for Tumor Growth Suppression. ACS Nano. 2021;15:19298–19309. doi: 10.1021/acsnano.1c05392. [DOI] [PubMed] [Google Scholar]
- 153.Zhang Y., Chen Y., Li J., Zhu X., Liu Y., Wang X., Wang H., Yao Y., Gao Y., Chen Z. Development of toll-like receptor agonist-loaded nanoparticles as precision immunotherapy for reprogramming tumor-associated macrophages. ACS Appl. Mater. Interfaces. 2021;13:24442–24452. doi: 10.1021/acsami.1c01453. [DOI] [PubMed] [Google Scholar]
- 154.Hu X.-X., He P.-P., Qi G.-B., Gao Y.-J., Lin Y.-X., Yang C., Yang P.-P., Hao H., Wang L., Wang H. Transformable nanomaterials as an artificial extracellular matrix for inhibiting tumor invasion and metastasis. ACS Nano. 2017;11:4086–4096. doi: 10.1021/acsnano.7b00781. [DOI] [PubMed] [Google Scholar]
- 155.Guo Z., Hu K., Sun J., Zhang T., Zhang Q., Song L., Zhang X., Gu N. Fabrication of hydrogel with cell adhesive micropatterns for mimicking the oriented tumor-associated extracellular matrix. ACS Appl. Mater. Interfaces. 2014;6:10963–10968. doi: 10.1021/am5023946. [DOI] [PubMed] [Google Scholar]
- 156.Grossman M., Ben-Chetrit N., Zhuravlev A., Afik R., Bassat E., Solomonov I., Yarden Y., Sagi I. Tumor Cell Invasion Can Be Blocked by Modulators of Collagen Fibril Alignment That Control Assembly of the Extracellular MatrixDisruption of Collagen Fibril Alignment Attenuates Cancer. Cancer Res. 2016;76:4249–4258. doi: 10.1158/0008-5472.CAN-15-2813. [DOI] [PubMed] [Google Scholar]
- 157.Zhou H., Fan Z., Deng J., Lemons P.K., Arhontoulis D.C., Bowne W.B., Cheng H. Hyaluronidase embedded in nanocarrier PEG shell for enhanced tumor penetration and highly efficient antitumor efficacy. Nano Lett. 2016;16:3268–3277. doi: 10.1021/acs.nanolett.6b00820. [DOI] [PubMed] [Google Scholar]
- 158.Pan A., Wang Z., Chen B., Dai W., Zhang H., He B., Wang X., Wang Y., Zhang Q. Localized co-delivery of collagenase and trastuzumab by thermosensitive hydrogels for enhanced antitumor efficacy in human breast xenograft. Drug Deliv. 2018;25:1495–1503. doi: 10.1080/10717544.2018.1474971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Du S., Xiong H., Xu C., Lu Y., Yao J. Attempts to strengthen and simplify the tumor vascular normalization strategy using tumor vessel normalization promoting nanomedicines. Biomater. Sci. 2019;7:1147–1160. doi: 10.1039/C8BM01350K. [DOI] [PubMed] [Google Scholar]
- 160.Li W., Zhao X., Du B., Li X., Liu S., Yang X.-Y., Ding H., Yang W., Pan F., Wu X. Gold nanoparticle–mediated targeted delivery of recombinant human endostatin normalizes tumour vasculature and improves cancer therapy. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep30619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gao W., Li S., Liu Z., Sun Y., Cao W., Tong L., Cui G., Tang B. Targeting and destroying tumor vasculature with a near-infrared laser-activated “nanobomb” for efficient tumor ablation. Biomaterials. 2017;139:1–11. doi: 10.1016/j.biomaterials.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 162.Satterlee A.B., Rojas J.D., Dayton P.A., Huang L. Enhancing nanoparticle accumulation and retention in desmoplastic tumors via vascular disruption for internal radiation therapy. Theranostics. 2017;7:253. doi: 10.7150/thno.16681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Doyle L.M., Wang M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8:727. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nedeva C., Mathivanan S. Engineering Extracellular Vesicles for Cancer Therapy. Subcell. Biochem. 2021;97:375–392. doi: 10.1007/978-3-030-67171-6_14. [DOI] [PubMed] [Google Scholar]
- 166.Elliott R.O., He M. Unlocking the Power of Exosomes for Crossing Biological Barriers in Drug Delivery. Pharmaceutics. 2021;13:122. doi: 10.3390/pharmaceutics13010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zitvogel L., Regnault A., Lozier A., Wolfers J., Flament C., Tenza D., Ricciardi-Castagnoli P., Raposo G., Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: Dendritic cell-derived exosomes. Nat. Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 168.Morse M.A., Garst J., Osada T., Khan S., Hobeika A., Clay T.M., Valente N., Shreeniwas R., Sutton M.A., Delcayre A., et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Besse B., Charrier M., Lapierre V., Dansin E., Lantz O., Planchard D., Le Chevalier T., Livartoski A., Barlesi F., Laplanche A., et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 2016;5:e1071008. doi: 10.1080/2162402X.2015.1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang J., Li W., Zhang L., Ban L., Chen P., Du W., Feng X., Liu B.-F. Chemically Edited Exosomes with Dual Ligand Purified by Microfluidic Device for Active Targeted Drug Delivery to Tumor Cells. ACS Appl. Mater. Interfaces. 2017;9:27441–27452. doi: 10.1021/acsami.7b06464. [DOI] [PubMed] [Google Scholar]
- 171.Jia G., Han Y., An Y., Ding Y., He C., Wang X., Tang Q. NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo. Biomaterials. 2018;178:302–316. doi: 10.1016/j.biomaterials.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 172.Li J., Luo Y., Li B., Xia Y., Wang H., Fu C. Implantable and Injectable Biomaterial Scaffolds for Cancer Immunotherapy. Front. Bioeng. Biotechnol. 2020;8:612950. doi: 10.3389/fbioe.2020.612950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lim S., Park J., Shim M.K., Um W., Yoon H.Y., Ryu J.H., Lim D.K., Kim K. Recent advances and challenges of repurposing nanoparticle-based drug delivery systems to enhance cancer immunotherapy. Theranostics. 2019;9:7906–7923. doi: 10.7150/thno.38425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cai L., Xu J., Yang Z., Tong R., Dong Z., Wang C., Leong K.W. Engineered biomaterials for cancer immunotherapy. MedComm. 2020;1:35–46. doi: 10.1002/mco2.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sinha A., Choi Y., Nguyen M.H., Nguyen T.L., Choi S.W., Kim J. A 3D Macroporous Alginate Graphene Scaffold with an Extremely Slow Release of a Loaded Cargo for In Situ Long-Term Activation of Dendritic Cells. Adv. Healthc. Mater. 2019;8:e1800571. doi: 10.1002/adhm.201800571. [DOI] [PubMed] [Google Scholar]
- 176.Chen Y., Zhang Y., Wang B., Fan Q., Yang Q., Xu J., Dai H., Xu F., Wang C. Blood Clot Scaffold Loaded with Liposome Vaccine and siRNAs Targeting PD-L1 and TIM-3 for Effective DC Activation and Cancer Immunotherapy. ACS Nano. 2023;17:760–774. doi: 10.1021/acsnano.2c10797. [DOI] [PubMed] [Google Scholar]
- 177.Ren L., Lim Y.T. Degradation-Regulatable Architectured Implantable Macroporous Scaffold for the Spatiotemporal Modulation of Immunosuppressive Microenvironment and Enhanced Combination Cancer Immunotherapy. Adv. Funct. Mater. 2018;28:1804490. doi: 10.1002/adfm.201804490. [DOI] [Google Scholar]
- 178.Cai M.-H., Chen X.-Y., Fu L.-Q., Du W.-L., Yang X., Mou X.-Z., Hu P.-Y. Design and Development of Hybrid Hydrogels for Biomedical Applications: Recent Trends in Anticancer Drug Delivery and Tissue Engineering. Front. Bioeng. Biotechnol. 2021;9:630943. doi: 10.3389/fbioe.2021.630943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liu M., Cao Z., Zhang R., Chen Y., Yang X. Injectable Supramolecular Hydrogel for Locoregional Immune Checkpoint Blockade and Enhanced Cancer Chemo-Immunotherapy. ACS Appl. Mater. Interfaces. 2021;13:33874–33884. doi: 10.1021/acsami.1c08285. [DOI] [PubMed] [Google Scholar]
- 180.Chen M., Tan Y., Dong Z., Lu J., Han X., Jin Q., Zhu W., Shen J., Cheng L., Liu Z., et al. Injectable Anti-inflammatory Nanofiber Hydrogel to Achieve Systemic Immunotherapy Post Local Administration. Nano Lett. 2020;20:6763–6773. doi: 10.1021/acs.nanolett.0c02684. [DOI] [PubMed] [Google Scholar]
- 181.Song H., Yang P., Huang P., Zhang C., Kong D., Wang W. Injectable polypeptide hydrogel-based co-delivery of vaccine and immune checkpoint inhibitors improves tumor immunotherapy. Theranostics. 2019;9:2299–2314. doi: 10.7150/thno.30577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Liu Y., Yan X., Zhang F., Zhang X., Tang F., Han Z., Li Y. TCR-T Immunotherapy: The Challenges and Solutions. Front. Oncol. 2021;11:794183. doi: 10.3389/fonc.2021.794183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Labanieh L., Majzner R.G., Mackall C.L. Programming CAR-T cells to kill cancer. Nat. Biomed. Eng. 2018;2:377–391. doi: 10.1038/s41551-018-0235-9. [DOI] [PubMed] [Google Scholar]
- 184.Grosskopf A.K., Labanieh L., Klysz D.D., Roth G.A., Xu P., Adebowale O., Gale E.C., Jons C.K., Klich J.H., Yan J., et al. Delivery of CAR-T cells in a transient injectable stimulatory hydrogel niche improves treatment of solid tumors. Sci. Adv. 2022;8:eabn8264. doi: 10.1126/sciadv.abn8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hu Q., Li H., Archibong E., Chen Q., Ruan H., Ahn S., Dukhovlinova E., Kang Y., Wen D., Dotti G., et al. Inhibition of post-surgery tumour recurrence via a hydrogel releasing CAR-T cells and anti-PDL1-conjugated platelets. Nat. Biomed. Eng. 2021;5:1038–1047. doi: 10.1038/s41551-021-00712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Stephan S.B., Taber A.M., Jileaeva I., Pegues E.P., Sentman C.L., Stephan M.T. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat. Biotechnol. 2015;33:97–101. doi: 10.1038/nbt.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liao J.B., Swensen R.E., Ovenell K.J., Hitchcock-Bernhardt K.M., Reichow J.L., Apodaca M.C., D’Amico L., Childs J.S., Higgins D.M., Buening B.J. Phase II trial of albumin-bound paclitaxel and granulocyte macrophage colony-stimulating factor as an immune modulator in recurrent platinum resistant ovarian cancer. Gynecol. Oncol. 2017;144:480–485. doi: 10.1016/j.ygyno.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 188.Sahin U., Oehm P., Derhovanessian E., Jabulowsky R.A., Vormehr M., Gold M., Maurus D., Schwarck-Kokarakis D., Kuhn A.N., Omokoko T. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585:107–112. doi: 10.1038/s41586-020-2537-9. [DOI] [PubMed] [Google Scholar]
- 189.Calmeiro J., Carrascal M., Gomes C., Falcão A., Cruz M.T., Neves B.M. Biomaterial-based platforms for in situ dendritic cell programming and their use in antitumor immunotherapy. J. ImmunoTherapy Cancer. 2019;7:1–11. doi: 10.1186/s40425-019-0716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sonneveld P., Hajek R., Nagler A., Spencer A., Bladé J., Robak T., Zhuang S.H., Harousseau J.L., Orlowski R.Z., Investigators D.M.S. Combined pegylated liposomal doxorubicin and bortezomib is highly effective in patients with recurrent or refractory multiple myeloma who received prior thalidomide/lenalidomide therapy. Cancer. 2008;112:1529–1537. doi: 10.1002/cncr.23326. [DOI] [PubMed] [Google Scholar]
- 191.Bonvalot S., Rutkowski P.L., Thariat J., Carrère S., Ducassou A., Sunyach M.-P., Agoston P., Hong A., Mervoyer A., Rastrelli M. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act. In. Sarc): A multicentre, phase 2–3, randomised, controlled trial. Lancet Oncol. 2019;20:1148–1159. doi: 10.1016/S1470-2045(19)30326-2. [DOI] [PubMed] [Google Scholar]
- 192.Gargett T., Abbas M.N., Rolan P., Price J.D., Gosling K.M., Ferrante A., Ruszkiewicz A., Atmosukarto I.I., Altin J., Parish C.R. Phase I trial of Lipovaxin-MM, a novel dendritic cell-targeted liposomal vaccine for malignant melanoma. Cancer Immunol. Immunother. 2018;67:1461–1472. doi: 10.1007/s00262-018-2207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Butts C., Maksymiuk A., Goss G., Soulieres D., Marshall E., Cormier Y., Ellis P.M., Price A., Sawhney R., Beier F. Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): Phase IIB randomized, multicenter, open-label trial. J. Cancer Res. Clin. Oncol. 2011;137:1337–1342. doi: 10.1007/s00432-011-1003-3. [DOI] [PubMed] [Google Scholar]
- 194.Kochenderfer J.N., Somerville R.P., Lu T., Yang J.C., Sherry R.M., Feldman S.A., McIntyre L., Bot A., Rossi J., Lam N. Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol. Ther. 2017;25:2245–2253. doi: 10.1016/j.ymthe.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Morgan R.A., Johnson L.A., Davis J.L., Zheng Z., Woolard K.D., Reap E.A., Feldman S.A., Chinnasamy N., Kuan C.-T., Song H. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum. Gene Ther. 2012;23:1043–1053. doi: 10.1089/hum.2012.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gauthier J., Bezerra E.D., Hirayama A.V., Fiorenza S., Sheih A., Chou C.K., Kimble E.L., Pender B.S., Hawkins R.M., Vakil A. Factors associated with outcomes after a second CD19-targeted CAR T-cell infusion for refractory B-cell malignancies. Blood. 2021;137:323–335. doi: 10.1182/blood.2020006770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Salvioni L., Rizzuto M.A., Bertolini J.A., Pandolfi L., Colombo M., Prosperi D. Thirty years of cancer nanomedicine: Success, frustration, and hope. Cancers. 2019;11:1855. doi: 10.3390/cancers11121855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nayak P.P., Narayanan A., Badekila A.K., Kini S. Nanomedicine in cancer clinics: Are we there yet? Curr. Pathobiol. Rep. 2021;9:43–55. doi: 10.1007/s40139-021-00220-6. [DOI] [Google Scholar]
- 199.Navya P., Kaphle A., Srinivas S., Bhargava S.K., Rotello V.M., Daima H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019;6:1–30. doi: 10.1186/s40580-019-0193-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Das K.P. Nanoparticles and Convergence of Artificial Intelligence for Targeted Drug Delivery for Cancer Therapy: Current Progress and Challenges. Front. Med.Technol. 2023;4:1067144. doi: 10.3389/fmedt.2022.1067144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data needed to support the conclusions are present in the paper. Additional data related to this paper may be requested from the authors.