Table 1.
Optimization of reaction conditions.
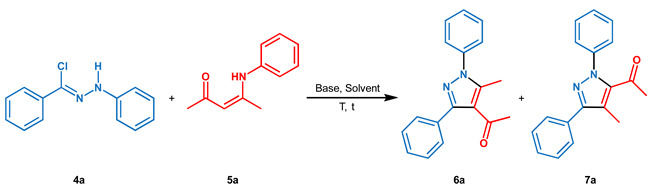
| Entry a | Solvent b | Base | T (°C) | t (h) | Yield (%) c | 6a:7a e |
|---|---|---|---|---|---|---|
| 1 | [mPy](OTf)/H2O | Et3N | rt | 2 | 50 | 90:10 |
| 2 | [mPy](OTf)/H2O | Et3N | rt | 24 | 51 | 80:20 |
| 3 | [mPy](OTf)/H2O | Et3N | 50 | 2 | 67 | 96:4 |
| 4 | [mPy](OTf)/H2O | Et3N | 85 | 2 | 32 | 95:5 |
| 5 | [mPy](OTf)/H2O | DBU | 50 | 2 | 42 | 96:4 |
| 6 | [mPy](OTf)/H2O | DMAP | 50 | 2 | 38 | 96:4 |
| 7 | [mPy](OTf)/H2O | NaOH | 50 | 2 | traces | - |
| 8 | [mPy](OTf)/H2O | K2CO3 | rt | 2 | 72 | 96:4 |
| 9 | [mPy](OTf)/H2O | K2CO3 | 50 | 2 | 85 | 96:4 |
| 10 d | [mPy](OTf)/H2O | K2CO3 | 50 | 2 | 90 | 97:3 |
| 11 d | [Bmim]Cl/H2O | K2CO3 | 50 | 2 | 75 | 95:5 |
| 12 d | [Bmim][BF4]/H2O | K2CO3 | 50 | 2 | 78 | 96:4 |
| 13 d | DMF | K2CO3 | 50 | 2 | 52 | 76:24 |
| 14 d | DMSO | K2CO3 | 50 | 2 | 64 | 82:18 |
a Reaction Conditions: enaminone 5a (1 eq), base (2.5 eq), hydrazonyl chloride 4a (1 eq) in 12 mL of solvent mixture. b IL/H2O 9:1 v:v; c isolated yield. d reaction conditions: enaminone 5a (1 eq), base (2.5 eq), hydrazonyl chloride 4a (1.3 eq) in 12 mL of solvent mixture. e regioisomeric ratio was determined by 1H NMR analysis of the crude reaction mixture (see Supplementary Materials).
