Table 2.
Synthesis of 1,3,4,5-tetrasubstituted pyrazoles by eliminative 1,3-dipolar cycloaddition.
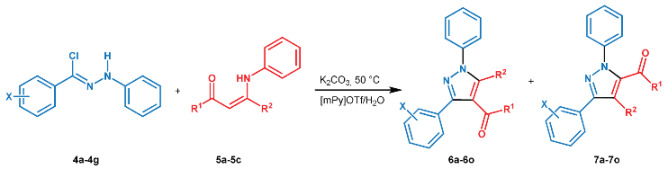
| Entry a | Hydrazonyl Chloride |
X | Enaminone | R1 | R2 | Product | t (h) |
Yield 6a–6o (%) b |
6:7 c |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4a | H | 5a | CH3 | CH3 | 6a | 2 | 90 | 97:3 |
| 2 | 4b | 4-OCH3 | 5a | CH3 | CH3 | 6b | 2 | 95 | 97:3 |
| 3 | 4c | 4-CH3 | 5a | CH3 | CH3 | 6c | 2 | 94 | 96:4 |
| 4 | 4d | 4-NO2 | 5a | CH3 | CH3 | 6d | 2 | 90 | 95:5 |
| 5 | 4e | 2-Cl | 5a | CH3 | CH3 | 6e | 2 | 92 | 96:4 |
| 6 | 4f | 3-Cl | 5a | CH3 | CH3 | 6f | 2 | 90 | 94:6 |
| 7 | 4g | 4-Cl | 5a | CH3 | CH3 | 6g | 2 | 92 | 98:2 |
| 8 | 4a | H | 5b | Ph | CH3 | 6h | 4 | 81 | 93:7 |
| 9 | 4b | 4-OCH3 | 5b | Ph | CH3 | 6i | 4 | 83 | 96:4 |
| 10 | 4c | 4-CH3 | 5b | Ph | CH3 | 6j | 4 | 82 | 96:4 |
| 11 | 4d | 4-NO2 | 5b | Ph | CH3 | 6k | 4 | 80 | 95:5 |
| 12 | 4e | 2-Cl | 5b | Ph | CH3 | 6l | 4 | 82 | 95:5 |
| 13 | 4f | 3-Cl | 5b | Ph | CH3 | 6m | 4 | 84 | 96:4 |
| 14 | 4g | 4-Cl | 5b | Ph | CH3 | 6n | 4 | 81 | 96:4 |
| 15 | 4a | H | 5c | Ph | Ph | 6o | 4 | 76 | 94:6 |
a Reaction conditions: enaminone 5a–5c (1 eq), K2CO3 (2.5 eq), hydrazonyl chloride 4a–4g (1.3 eq) in 12 mL of solvent mixture. b isolated yield. c regioisomeric ratio values were determined by 1H NMR analysis of the crude reaction mixture (see Supplementary Materials).
