Figure 5.
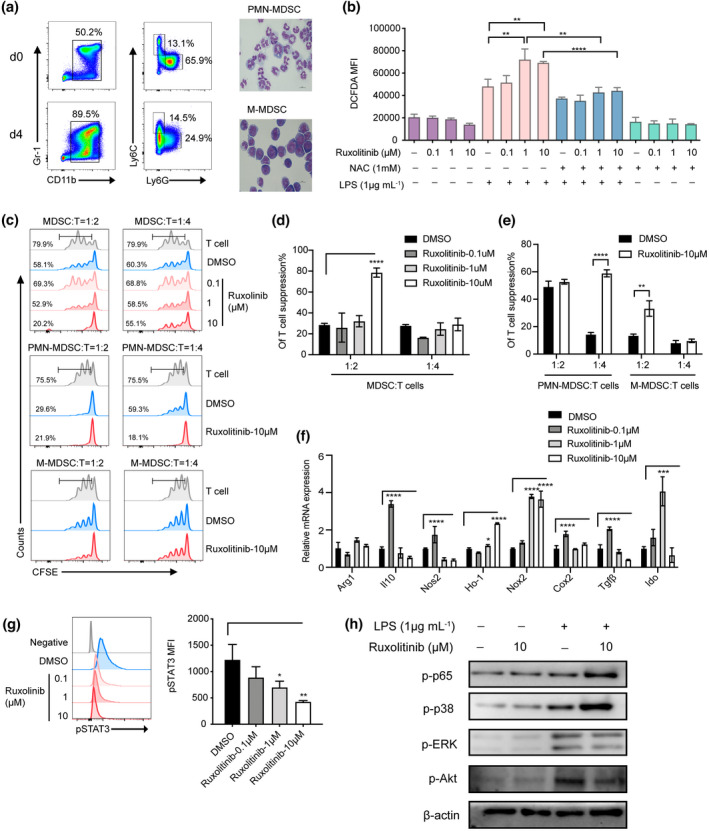
Ruxolitinib‐pretreated PMN‐MDSCs displayed remarkable immunosuppressive function by upregulation of Nox2 to regulate ROS generation via bypass activating NF‐κB/MAPK‐p38 pathways in vitro. MDSCs were generated in vitro from BM cells of C57BL/6 mice in the presence of 40 ng mL−1 GM‐CSF and IL‐6. After 4 days, cells were stained for CD11b and Gr‐1 expression or the distribution of MDSC subsets was gated on CD11b+ cells by the expression of Ly6C and Ly6G. (a) Data show one representative flow cytometric analysis and morphology of MDSCs subsets. (b) In vitro purified PMN‐MDSCs were pretreated with or without ruxolitinib (0.1 μm, 1 μm, 10 μm) for 2 h and then incubated with LPS (1 μg mL−1) in the presence or absence of ROS inhibitor NAC (1 mm) for another (h). The production of ROS was monitored by DCFDA flow cytometry in each group (n = 3). (c–e) CFSE‐labelled CD3+ T cells (1 × 105 per well) were stimulated by CD3/28 beads, then in vitro induced MDSCs, PMN‐MDSCs and M‐MDSCs were added at different ratios with or without ruxolitinib pretreated cocultured for 72 h. Proliferation of CFSE‐labelled CD3+ T cells was measured with flow cytometry. In vitro purified PMN‐MDSCs were pretreated with or without ruxolitinib (0.1 μm, 1 μm,10 μm) for 2 h and then stimulated with LPS (1 μg mL−1) (n = 3). (f) The immunosuppressive molecules of PMN‐MDSCs were detected by real‐time PCR (n = 3). The expression of pSTAT3, p‐p65, p‐p38, p‐ERK and p‐Akt were examined by phosflow analysis (g) and western blot assay (h). Data are expressed as mean ± standard error (SE). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. These results are representative of three independent experiments.
