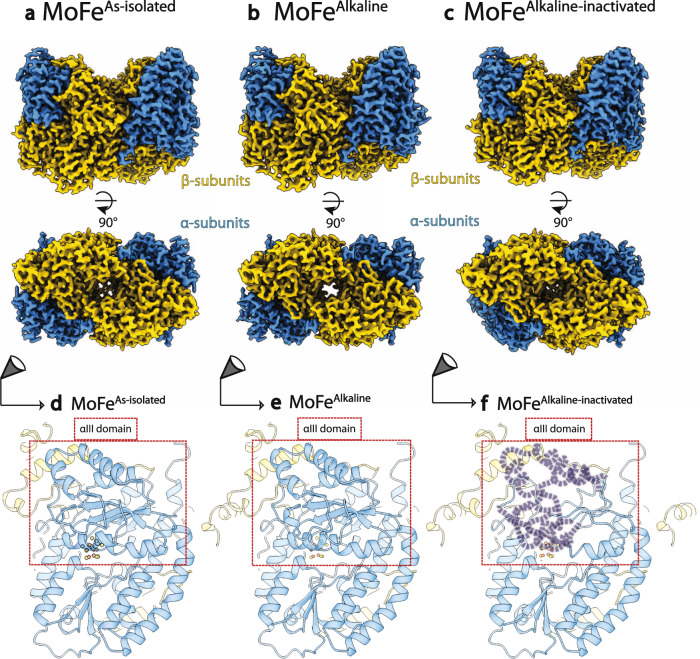
Fig. 1. Anaerobic cryoEM structures of MoFe-protein.
a The 2.13 Å resolution cryoEM map of MoFeAs-isolated purified from A. vinelandii. b The 2.14 Å resolution cryoEM map of MoFeAlkaline from a control acetylene reduction reaction performed at pH 9.5 without ATP isolated via S.E.C. conducted at pH 7.8, demonstrating the same overall architecture as MoFeAs-isolated. c The 2.37 Å resolution cryoEM map of MoFeAlkaline-inactivated from an acetylene reduction reaction performed at pH 9.5 with ATP isolated via S.E.C. conducted at pH 7.8, displaying asymmetric disorder within the α-subunit density. d–f Location of disorder within the αIII domain of the α-subunit in the d MoFeAs-isolated, e MoFeAlkaline, and f MoFeAlkaline-inactivated structures. α-subunits are illustrated in blue and β-subunits are illustrated in yellow. Structures were solved in the presence of CHAPSO to prevent interactions with the air–water interface.