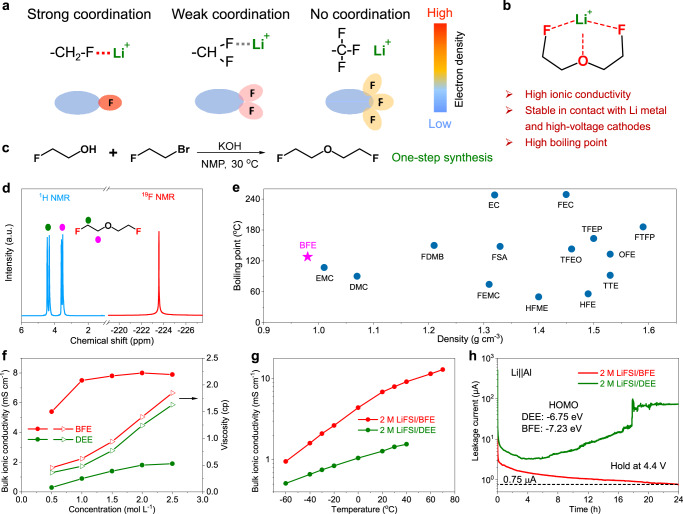
Fig. 1. Physicochemical and electrochemical characterizations of the monofluoride ether-based electrolyte with tridentate coordination chemistry.
a Coordination chemistry of monofluoride, difluoro, and trifluoro groups. b The molecular design of the BFE, can coordinate Li+ ions with one Li-O and two Li-F interactions simultaneously. c One-step synthesis of BFE solvent at 30 °C. d 1H, and 19F NMR spectra of BFE molecule. e Boiling point and density of BFE and conventional fluorinated/non-fluorinated solvents at 30 °C. f Bulk ionic conductivities, and viscosities of BFE and DEE electrolytes with various salt concentrations at 30 °C. g Measured bulk ionic conductivity of BFE and DEE electrolytes as a function of temperatures. h Leakage currents of Li||Al cells using BFE and DEE electrolytes at a constant applied voltage of 4.4 V and a temperature of 30 °C. Insert shows the HOMO levels for DEE and BFE, respectively.