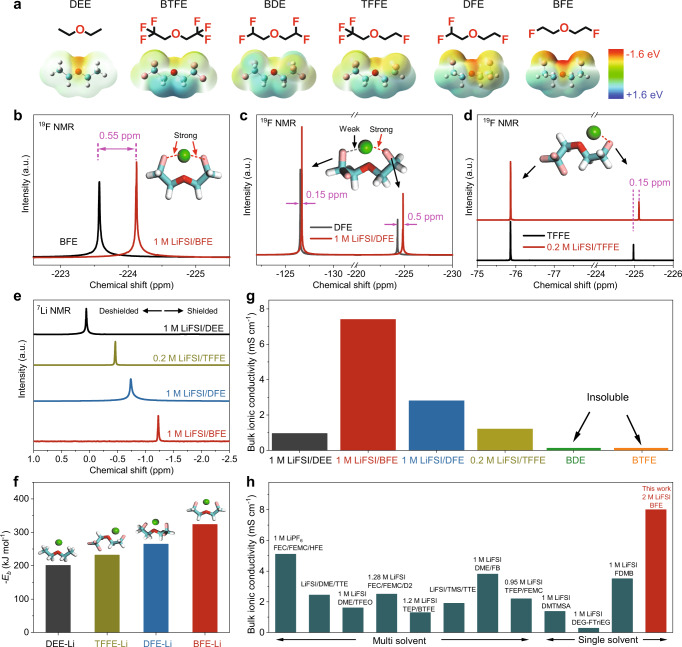
Fig. 2. Structure-property relationships between fluorination degree and ionic conductivity.
a Comparison of chemical structures and electrostatic potentials (ESP) of various ether solvents including DEE, BTFE, BDE, TFFE, DFE, and BFE. b–d 19F NMR of BFE, DFE, and TFFE before and after the salt dissolution (Reference: CF3COOH). Green sphere represents Li+ ion, while sticks in white, pink, light blue and red color represent H, F, C, and O atoms, respectively. e 7Li NMR of LiFSI salts in different electrolytes. f Solvating energies of DEE, TFFE, DFE, and BFE electrolytes. g Bulk ionic conductivities of various electrolytes at 30 °C, including DEE, BTFE, BDE, TFFE, DFE, and BFE. h Bulk ionic conductivity of BFE and previously reported fluorinated electrolytes at 30 °C. Because LiFSI is almost insoluble in BTFE and BDE, the bulk ionic conductivity value is not reported in the chart.