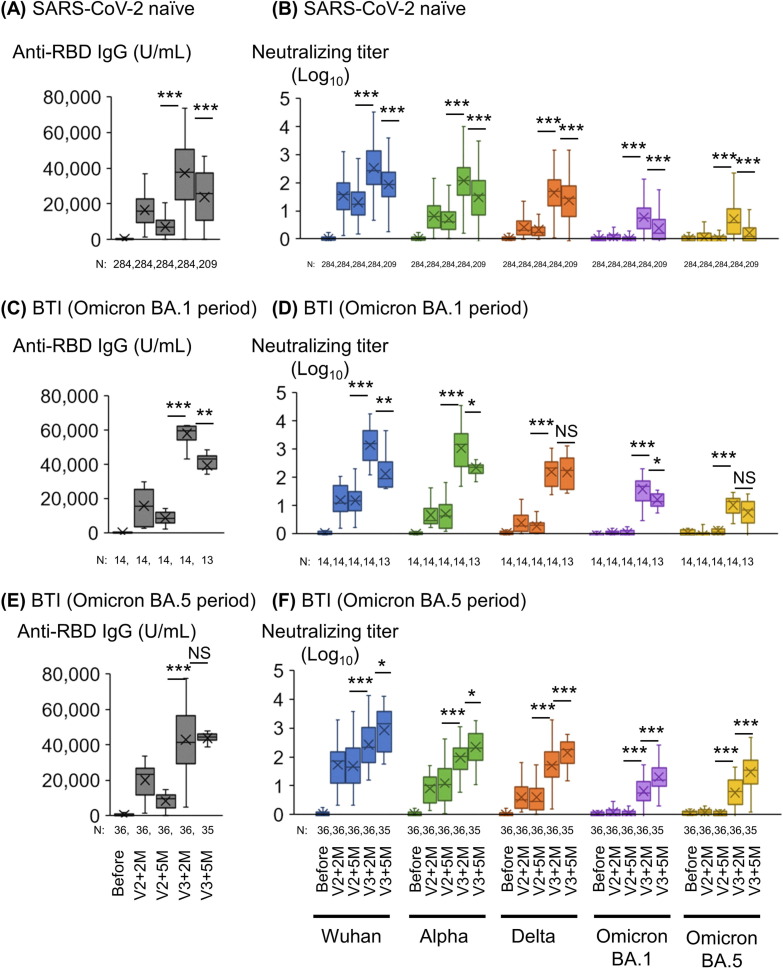
Fig. 2.
Anti-RBD IgG titers and neutralizing titers at 2 and 5 months after two doses and the third dose of mRNA vaccine in participants with or without BTI. (A) SARS-CoV-2 RBD IgG antibody titers, (B) Neutralizing titers against SARS-CoV-2pv of SARS-CoV-2 naïve participants. (C) SARS-CoV-2 RBD IgG antibody titers, (D) Neutralizing titers of participants with BTI during BA.1 epidemic period (January to March 2022). (E) SARS-CoV-2 RBD IgG antibody titers, (F) Neutralizing titers of participants with BTI during BA.5 epidemic period (June to September 2022). 14 participants were infected from January to March 2022 (during the Omicron BA.1 epidemic period); and 36 participants were infected from June to September 2022 (during the Omicron BA.5 epidemic period). ***, p < 0.001; **, p < 0.01; *, p < 0.05; NS, not significant. Abbreviations: RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; V2, 2nd dose of vaccine; V2 + 2 M, 2 months after the 2nd dose of vaccine; V2 + 5 M, 5 months after the 2nd dose of vaccine; V3, 3rd dose of vaccine; V3 + 2 M, 2 months after the 3rd dose of vaccine; V3 + 5 M, 5 months after the 3rd dose of vaccine; BTI, breakthrough infection.