Table 1.
The Pharmacokinetics of MRAs.
| Agent | Spironolactone | Eplerenone | Finerenone | Esaxerenone | Apararenone | Ocedurenone | Miricorilant | Balcinrenone | Drospirenone/Estetrol | Canrenone |
|---|---|---|---|---|---|---|---|---|---|---|
| Name | SC 9420 | BAY 94-8862 | CS-3150 | MT-3995 | KBP-5074 | CORT-118335 | AZD-9977 | SC 9376 | ||
| Dose | 10mg/20mg | 25mg/50mg | 10mg/20mg | 1.25mg/2.5mg/5mg | 2.5mg/5mg/10mg | 0.25mg/0.5mg | in development | in development | ||
| Company | Pfizer | Pfizer | Bayer | Daiichi-Sankyo Company Limited, Japan | Mitsubishi Tanabe Pharma Corporation | KBP Biosciences | Corcept, Argenta Discovery | AstraZeneca | Mithra Pharmaceuticals, Estetra S.A., Libbs | |
| Generation of MRA | first | second | third | third | MRA | MRA | GRA; MRA | MR regulator | MRA; PR agonist; ARA; selective ER regulator | first |
| Steroidal/Nonsteroidal | steroidal | steroidal | nonsteroidal | nonsteroidal | nonsteroidal | nonsteroidal | nonsteroidal | nonsteroidal | steroidal | |
| Molecular Formular | C24H32O4S | C24H3O6 | C21H22N4O3 | C22H21F3N2O4S | C17H17FN2O4S | C28H30ClN5O2 | C24H23F3N2O2 | C20H18FN3O5 | C24H30O3 | C22H28O3 |
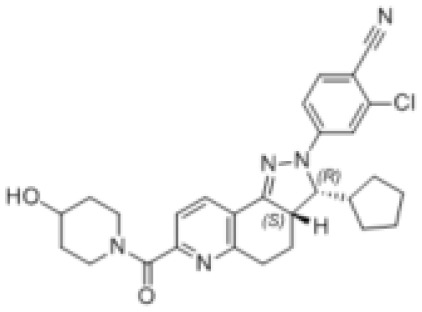
| Structure |

|

|

|

|

|
|

|

|

|

|
| Characteristic | potent and unselective | less potent and more selective than spironolactone | more potent and more selective than spironolactone | more potent and more selective than eplerenone | more potent and more selective than spironolactone | more potent and more selective; moderate affinity to MR | no affinity to GR/PR/AR | |||
| Heart-kidney distribution ratio | 1:6 | 1:3 | 1:1; cannot across the Blood-Brain Barrier | 1:1 | ||||||
| t½ | 13~24h(qd/bid); 9~16hqid | 4-6h | 1.7-2.8h | 20-30h | 275-285h for parent drug;>1000h for active metabolite | increased with dose | 31h | |||
| Tmax | 2.6-3.05h | 1.5h | 0.75-1h | 1.5-4h | 4h | 0.5-0.8h | 1-2h | |||
| Cmax | 209-301ng/ml | – | 160ug/L(20mg) | 37ng/ml | ||||||
| MR IC50 | 24 | 990 | 17.8 | moderate affinity to MR | ||||||
| AR IC50 | 77 | ≥21240 | ≥10000 | almost no affinity to GR/PR/AR | almost no affinity to GR/AR | |||||
| GR IC50 | 2410 | ≥21980 | ≥10000 | |||||||
| PR IC50 | 740 | ≥31210 | ≥10000 | |||||||
| Oral bioavailability | >90% | 69% | 86.50% | 76-85% | ||||||
| Protein binding ratio | >90% | 33%-60% | 92% | 95-97% | ||||||
| Metabolism | prodrug with multiple active metabolites | no active metabolites | no active metabolites | n/a | metabolite with low activity (MR binding affinity one-fiftieth of that of apararenone) | n/a | the active metabolites of spironolactone | |||
| Hyperkalemia | high risk | high risk | low risk | low risk | no risk | |||||
| Excretion | <1% unchanged drug recovered in urine; 10-15% of dose excreted in urine form of metabolites | 66% of dose excreted via urine; <3% unchanged drug recovered from urine | 80% of dose excreted via urine; <1% unchanged drug excreted in urine | 38.5% of dose excreted in urine; <2% unchanged drug excreted in urine | <14% of dose excreted in urine | 24–37% of dose excreted in urine; 20% of dose excreted unchanged in urine | ||||
| Sex-like ADR | common | less than spironolactone | no statistics difference with placebo group | |||||||
| Dose adjust based on renal function | excretion through the kidney | cannot be removed by hemodialysis | decrease dose in patients with eGFR≤60 and prohibit when eGFR<25 | decrease dose in patients with eGFR 30-50 | ||||||
| Indication | PA; HBP; hypokalemia; edema; HF | congestive heart failure; HBP | T2DM with CKD, ESRD, CVD, congestive heart failure | HBP, DKD(clinical trial phase) | DKD | HBP, DKD, HN | obesity; prostate cancer; metabolism disorder | DKD | contraception | HBP |
| Contraindication | not recommended to CRF | not recommended to CRF |
The unit of IC50 is nmol/L. the unit of eGFR is ml/min/1.73m2. PR, progesterone receptor. ER, estrogen receptor. AR, androgen receptor. MRA, mineralocorticoid receptor antagonist. t½, geometric mean terminal half-life. Tmax, median time to maximum plasma concentration. Cmax, maximum plasma concentration. ADR, adverse drug reactions. eGFR, estimated glomerular filtration rate. PA, primary aldosteronism. HBP, hypertension. CKD, chronic kidney diseases. DKD, diabetic kidney diseases. HN, hypertensive nephropathy. ESRD, end-stage renal disease.
