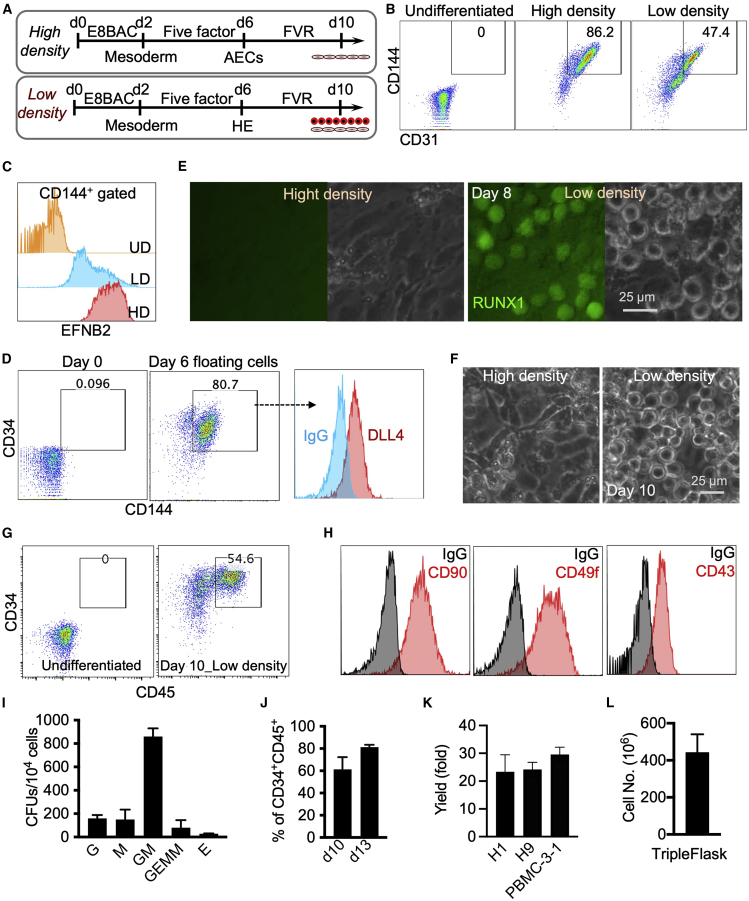
Figure 1.
Low-density arterial endothelium differentiation cultures allow for the generation of blood cells
H9 cells are used unless specified.
(A) Schematic of arterial endothelial cell and hematopoietic cell differentiation. High cell density, 1.1 × 105 cells/cm2. Low cell density, 1.8 × 104 cells/cm2. FVR media contains FGF2, VEGFA, and RESV.
(B) Representative flow cytometry dot plots show expression of CD31 and CD144 at day 6 of differentiation in low- and high-density conditions. Undifferentiated cells (day 0) are used as a negative control.
(C) CD144+ cells in low density (LD) and high density (HD) were gated for analysis of EFNB2 expression. Undifferentiated cells (UDs) are used as a negative control. The EFNB2-tdTomato/EPHB4-EGFP H1 reporter cell line was used.
(D) Representative flow cytometry analysis of CD144, CD34, and DLL4 expression of floating cells collected at day 6 of differentiation.
(E) RUNX1+23 enhancer activity in LD and HD cultures at day 8 of differentiation. The RUNX1+23 enhancer-Venus reporter cell line was used.
(F) Phase contrast images of arterial endothelium cultures at day 10 of differentiation.
(G) Representative flow cytometry analysis of CD34 and CD45 expression at day 10 of differentiation.
(H) Representative flow cytometry analysis of CD90, CD49f, and CD43 expression of floating cells collected at day 10 of differentiation.
(I) Colony-forming unit assay of day 8 cells in low cell density condition. Data are represented as mean ± SD; n = 3 independent experiments.
(J) Percentages of CD34+CD45+ cells at days 10 and 13 of differentiation. Data are represented as mean ± SD. Student’s t test; ∗p < 0.05; n = 3 independent experiments.
(K) Hematopoietic cells generated from one starting hPSC. H1 and H9 embryonic stem cells and PBMC-3-1 hiPSCs were used. Data are represented as mean ± SD; n = 3 independent experiments.
(L) Total floating hematopoietic cell number generated from one T500 TrypleFlask. Data are represented as mean ± SD; n = 3 independent experiments.