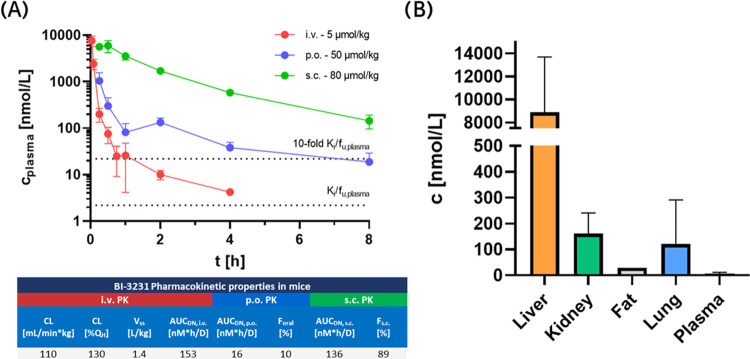
Figure 6.
In vivo pharmacokinetics and tissue distribution of 45 (BI-3231) in mice (n = 3, standard deviation (SD) indicated by error bars). (A) Plasma pharmacokinetics after intravenous and oral administration in mice was characterized by a biphasic and rapid plasma clearance that exceeded the hepatic blood flow and a low oral bioavailability of 10%. Bioavailability was significantly increased by subcutaneous dosing. Relevant systemic exposure corresponding to >10-fold in vitro mouse Ki in unbound plasma concentration could be maintained over 8 h in mice. (B) Tissue exposure 1 h after i.v. administration indicated extensive hepatic accumulation compared to plasma and other tissues, despite comparable in vitro tissue binding properties (PPB = 77.5%, TBliver = 87.1%, TBkidney = 77.8%, TBlung = 70.4%).