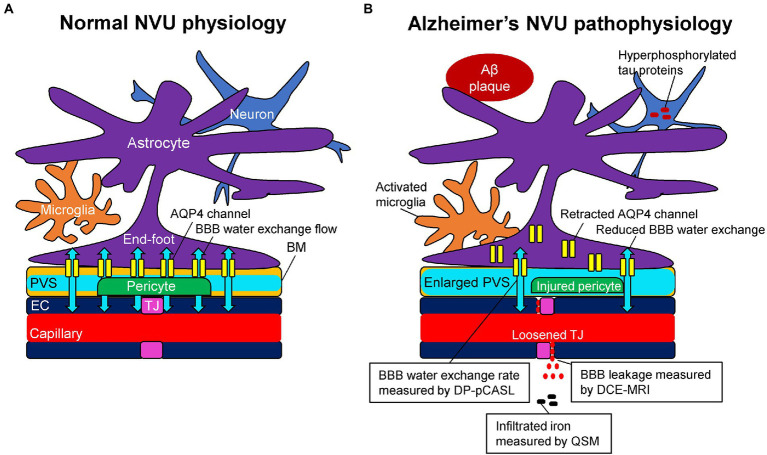
Figure 1.
Schematic representation of the neurovascular unit (NVU) in normal physiology (A) and Alzheimer’s pathophysiology (B). (A) The capillary lumen is formed by endothelial cells (EC), pericytes embedded in the basement membrane (BM), and astrocytic end-feet. Tight junction (TJ) proteins seal the ECs together and restrict the passage of solutes into the brain. The BM are separated into two layers, which are attached to the ECs and astrocytic feet, forming the perivascular space (PVS). Aquaporin-4 (AQP4) water channels expressed in the astrocytic end-feet membranes facilitate bidirectional water flow between the capillary, PVS, and interstitial tissues, resulting in the glymphatic flow. (B) In Alzheimer’s pathophysiology, the damaged TJ proteins loosen the seal of the ECs and provide a route for passive diffusion of toxic substances into the extravascular space, which can further damage or downregulate the NVU system. As a consequence of the injured pericyte that normally anchors the AQP4 water channels to astrocytic end-feet, the retraction of AQP4 from astrocytic end-feet membranes occurs with a reduction of bidirectional water flow, resulting in an enlarged PVS. Based on these pathophysiological changes, dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) can measure the blood–brain barrier (BBB) leakage, pseudo-continuous diffusion-prepared arterial spin labeling (DP-pCASL) can estimate the BBB water exchange rate, and quantitative susceptibility mapping (QSM) can detect the brain iron concentration, respectively.