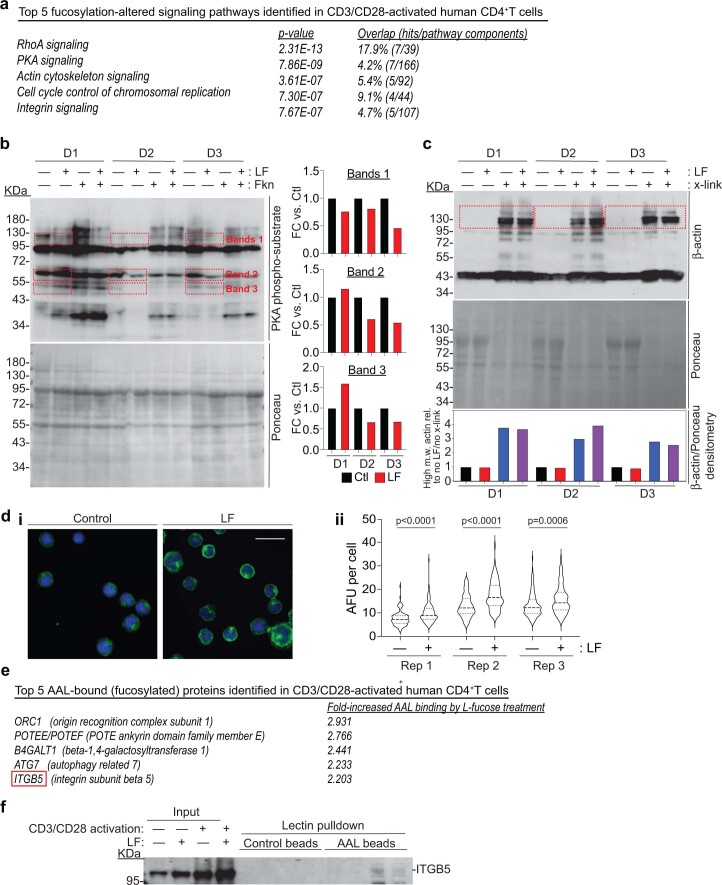
Extended Data Fig. 2. Fucosylation of CD4+T cells affects PKA activity and actin polymerization; and the identification of Integrin β5 as a highly fucosylated protein in activated CD4+T cells.
(a) Top 5 pathways in human CD4+T cells that are affected by increased fucosylation identified by Ingenuity Pathway Analysis (Qiagen; pathways were identified from phosphoproteomic analyses of CD3/CD28-activated human PBMC-derived CD4+T cells treated ± 250 µM L-fucose (LF) isolated from 3 independent healthy human donors (D1, D2, D3)). (b) (left) Immunoblot of PKA phosphorylated substrates (top) and Ponceau staining (bottom) of human PBMC-derived, CD3/CD28-activated CD4+T cells that were treated ± 250 µM LF (for 72 h) ± 10 µM forskolin (Fkn, a PKA agonist; for 6 h). (right) Densitometric quantification of selected bands (red dashed boxes) normalized to Ponceau staining. (c) Immunoblot of β-actin (top) and Ponceau staining (middle) of 3 human donor PBMC-derived, CD3/CD28 activated CD4+T cells were treated ± 250 µM LF ± 25 mM DTSP crosslinker (x-link). (bottom) Densitometric quantification of high molecular weight β-actin oligomers (red dashed boxes) normalized to Ponceau intensity and then normalized to -LF, -x-link samples. (d) (i) Coverslip-grown Jurkat cells treated ± 250 µM LF (for 72 h), fixed, and stained with DAPI (blue) and phalloidin-488 (green) for actin cytoskeleton. Scale bar: 25 µm. (ii) Integrated actin signal densities per cell from 3 biological replicates of Jurkat cells grown/treated as in (i) were measured using ImageJ v1.53a (NIH) and plotted as shown using GraphPad Prism v8. (e) Top 5 AAL-bound (fucosylated) proteins in human PBMC-derived, CD3/CD28-activated CD4+T cells from (a) that were identified by Ingenuity Pathway Analysis (Qiagen). (f) Of the 5 top hits, we were only able to validate fucosylation of Integrin β5 by LPD analysis of human PBMC-derived, CD3/CD28-activated CD4+T cells.