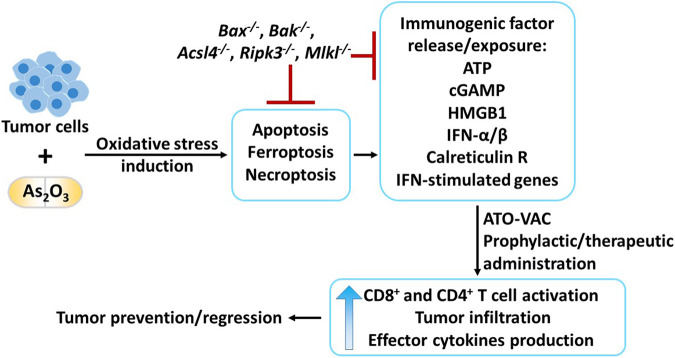
Fig. 1.
Arsenic trioxide (As2O3, ATO)-induced immunogenic cell death in tumors as a tool to elicit antitumor immunity. Whole-cell tumor vaccines generated using cytotoxic drugs ex vivo offer an attractive approach to target virus-irrelevant neoplasms. Applying this knowledge, Chen et al. explored the prophylactic and therapeutic efficacy of an ATO-based whole-cell vaccine. Preconditioning of tumor cells with ATO induces oxidative stress, which, simultaneously or sequentially, triggers cell death pathways such as apoptosis, ferroptosis and necroptosis. These pathways are associated with the release or exposure of numerous immunogenic factors that activate the immune system. Deletion of executors of each of the involved death pathways inhibited tumor cell death and thus blocked the release of immunogenic factors. The prophylactic and therapeutic administration of an ATO-based whole-cell vaccine (ATO-VAC) successfully induced an adaptive immune response. Thus, a strong antitumor response in the recipient mice was elicited by creating a highly immunogenic tumor microenvironment that was flooded with activated tumor-infiltrating CD8+ and CD4+ T cells with heightened effector cytokine production, leading to tumor prevention or regression