Abstract
Background
Heterogeneity in clinical bleeding phenotype has been observed in hemophilia patients with similar FVIII or FIX activity levels. Thrombin generation and plasmin generation, as a global hemostasis assay, may contribute to a better prediction of which patients are at an increased risk of bleeding.
Objectives
The objective of this study was to describe the association between clinical bleeding phenotype and thrombin generation and plasmin generation profiles in patients with hemophilia.
Methods
The Nijmegen Hemostasis Assay, which simultaneously measures thrombin and plasmin generation, was performed in plasma samples of patients with hemophilia participating in the sixth Hemophilia in the Netherlands study (HiN6). Patients receiving prophylaxis underwent a washout period. A severe clinical bleeding phenotype was defined as a self-reported annual bleeding rate of ≥5, a self-reported annual joint bleeding rate of ≥3, or the use of secondary/tertiary prophylaxis.
Results
In total, 446 patients, with a median age of 44 years, were included in this substudy. Thrombin generation and plasmin generation parameters differed between patients with hemophilia and healthy individuals. The median thrombin peak height was 1.0 nM, 25.9 nM, 47.1 nM, and 143.9 nM in patients with severe, moderate, and mild hemophilia and healthy individuals, respectively. A severe bleeding phenotype was observed in patients with a thrombin peak height of <49% and a thrombin potential of <72% compared to healthy individuals, and was independent of the hemophilia severity. The median thrombin peak height was 0.70% in patients with a severe clinical bleeding phenotype and 30.3% in patients with a mild clinical bleeding phenotype. The median thrombin potentials for these patients were 0.06% and 59.3%, respectively.
Conclusion
A decreased thrombin generation profile is associated with a severe clinical bleeding phenotype in patients with hemophilia. Thrombin generation in combination with bleeding severity may be a better tool to personalize prophylactic replacement therapy irrespective of hemophilia severity.
Keywords: factor VIII, hemophilia A, hemophilia B, phenotype, thrombin
Essentials
-
•
Hemophilia patients with similar factor activity levels may have different bleeding phenotypes.
-
•
Thrombin generation (TG) parameters were obtained for 446 patients with hemophilia.
-
•
A severe phenotype is associated with a decreased TG profile, independent of hemophilia severity.
-
•
The TG profile and bleeding severity hold promise to be used to personalize prophylactic therapy.
1. Introduction
Hemophilia is an X-linked hereditary bleeding disorder due to an abnormally low activity level of coagulation factor VIII (FVIII; in hemophilia A) or factor IX (FIX; in hemophilia B). The severity of the disease is defined by the residual activity level of the deficient factor (severe, <1%; moderate, 1%-5%; and mild, >5%-40%) and generally correlates with the clinical bleeding phenotype. Patients with severe hemophilia typically suffer from spontaneous bleeding in joints and muscles, whereas patients with moderate or mild hemophilia mainly develop bleeds after trauma or surgery [1].
Interestingly, there is increasing evidence that baseline FVIII or FIX activity levels do not correlate as well with the clinical bleeding phenotype as expected. Ten to fifteen percent of patients with severe hemophilia show a mild clinical bleeding tendency [2]. On the other hand, a distinct group of patients with moderate hemophilia suffer from spontaneous joint bleeding. Furthermore, several studies reported a variability in bleeding frequency, requirement of replacement factor concentrates, age of first bleed, and joint damage in patients with similar factor activity levels [2]. Therefore, using FVIII or FIX activity levels to guide prophylactic replacement therapy, as is recommended by current guidelines [1], may be suboptimal.
A more useful tool to differentiate between bleeding phenotypes in patients with hemophilia and eventually to guide prophylactic replacement therapy may be the thrombin generation (TG) profile. Studies using thrombin generation assays (TGAs) found a correlation between TG parameters and the factor activity level [[3], [4], [5], [6], [7], [8], [9], [10]]. In addition, previous studies suggested that the TG profile better determines which hemophilia patients are at increased risk of bleeding [3,4,7,8,[11], [12], [13], [14], [15], [16], [17]]. A decreased thrombin peak height and thrombin potential were found in patients with a severe clinical bleeding phenotype irrespective of the FVIII or FIX activity level. Furthermore, several studies demonstrated that the guidance and monitoring of prophylactic replacement therapy could be optimized using TG parameters alongside factor activity levels [[18], [19], [20]].
However, these studies had 2 main limitations. First of all, they were conducted among a limited group of patients. Second, the outcomes were difficult to generalize because they were performed using different TGAs under different preanalytical conditions. In addition, none of these studies investigated the association between plasmin generation (PG) and the clinical bleeding phenotype, even though previous research showed an enhanced fibrinolytic capacity in patients with low TG [10,21,22].
Therefore, the aim of this cross-sectional study was to accurately describe the association between the clinical bleeding phenotype and individual TG and PG profiles. To accomplish this, the Nijmegen Hemostasis Assay (NHA) was measured in a cohort of 446 patients with hemophilia who participated in the sixth Hemophilia in the Netherlands (HiN6) study. The NHA was developed to simultaneously measure TG and PG [23].
2. Methods
The HiN6 study (registered at the Dutch Trial Register; NL59114.058.17) is a nationwide cross-sectional study conducted among patients with congenital hemophilia A (HA) or hemophilia B (HB) and includes patients from all 6 Dutch hemophilia treatment centers (HTCs) from June 2018 to July 2019. Patients of all ages with severe, moderate, or mild hemophilia were invited to participate. The study consisted of data collection from the electronic patient file, a questionnaire, and blood and urine sampling. The study design of the HiN6 has been described previously [24]. This study was approved by the Medical Ethical Committee of Leiden University Medical Center. All patients and/or parents, in case of minors, provided written informed consent.
2.1. Data collection
The following data were collected from the electronic patient file: age, type and severity of hemophilia, treatment regimen (prophylactic or on-demand treatment and type and dosage of the [non-]replacement concentrate), inhibitor status, and diagnosis of a concomitant coagulation disorder (ie, von Willebrand disease, thrombocytopathy, or coagulation factor deficiency). The term child was used for an individual aged <18 years at the time of inclusion, and the term adult for an individual aged ≥18 years at the time of inclusion. Hemophilia severity was defined by the baseline FVIII or FIX activity level: severe, <1%; moderate, 1% to 5%; and mild, >5% to 40%.
Answers to the self-reported questionnaire were used to determine if patients used primary or secondary/tertiary prophylaxis. The terms primary, secondary, and tertiary prophylaxis were defined according to when and why the prophylactic treatment was initiated [1]. Patients reporting a starting age of <3 years were considered to have primary prophylaxis. Furthermore, the self-reported questionnaires were used to calculate the (annual; joint) bleeding rate. Patients were asked to only report the bleeding episodes that required administration of DDAVP, FVIII, or FIX concentrates for ≥2 days. For adults, the annual (joint) bleeding rate (A(J)BR) was defined as the number of self-reported (joint) bleeds in the previous 12 months. Adults with an ABR of ≥5 and/or an AJBR of ≥3 and/or adults using secondary/tertiary prophylaxis were classified as having a severe clinical bleeding phenotype. Adults with an ABR of <5 and an AJBR of <3, who did not use prophylaxis, were classified as having a mild clinical bleeding phenotype. For children, the (joint) bleeding rate [(J)BR] was determined based on the results of the preceding 3 months. Children with a BR of ≥2 and/or a JBR of ≥1 and/or children using secondary prophylaxis were classified as having a severe clinical bleeding phenotype. Children with a BR of <2 and a JBR of <1, who did not use prophylaxis, were classified as having a mild clinical bleeding phenotype. Patients using primary prophylaxis were not classified into having a mild or a severe clinical bleeding phenotype as their actual bleeding tendency is unknown.
2.2. Blood collection and processing
Blood samples were collected during a regular visit to the HTC at least 3 days after last FVIII administration and at least 5 days after last FIX administration. Patients on replacement regime for a breakthrough bleeding (spontaneous or after trauma) or perioperatively were excluded from blood sampling. The date and time of blood sampling, date and time of last FVIII or FIX infusion before blood sampling, and the specific (non)replacement product and dosage were reported. Patients who had not fulfilled the washout period or patients using an extended half-life product, a bypassing agent (activated prothrombin complex concentrate [aPCC] or activated recombinant FVII [rFVIIa]), or a nonreplacement product (emicizumab) were excluded from TG and PG analysis in this study. When information on the washout period was missing, the FVIII or FIX activity level was determined. In case of an activity level that did not correspond to the patient’s hemophilia severity, it was assumed that the washout period was not completed, and consequently, the patient was excluded from analysis of the TG and PG profiles.
Blood samples were processed immediately after collection at each treatment center. Platelet-poor plasma (PPP) was obtained for NHA measurements. Samples were centrifuged for 15 minutes at 3000 g and room temperature. The PPP samples were then aliquoted into 1.5-mL long-term freezer storage tubes with O-ring screw caps and stored at −80 °C. The samples were sent on dry ice to the Radboud university medical center, Nijmegen, the Netherlands, for central measurement of the NHA.
2.3. Factor VIII and factor IX activity level assay
FVIII and FIX activity levels were determined in the Radboud university medical center, Nijmegen, the Netherlands, using the FVIII and FIX one-stage clotting assay (Cephascreen reagents and STA Evolution, Stago Group) according to standard diagnostic procedures.
2.4. Nijmegen Hemostasis Assay
The NHA simultaneously measures TG and PG in a single well. The method of the assay has been described previously [23,25]. In short, TG was initiated by a low (0.28 pM) tissue factor (TF) concentration. PG was initiated by tissue plasminogen activator (tPA) and is dependent on fibrin formation. After adding a thrombin- and plasmin-specific fluorescent substrate, the proteolytic activity of both thrombin and plasmin was measured.
Seven NHA parameters were obtained after differentiation of the raw signals: (1) lag time, (2) time to thrombin peak, (3) thrombin peak height and (4) thrombin potential for the TG profile, (5) fibrin lysis time (FLT), (6) plasmin peak height, and (7) plasmin potential for the PG profile [23]. All parameters of the NHA were determined by a Microsoft Excel macro program in Microsoft Visual Basic (version 11.1.1 [Microsoft Corporation]). When TG or PG was minimal, the macro was not able to determine the parameters accurately. In these cases, the lag time and time to thrombin peak were set to 140 minutes (2 times the total time period of the measurement of the NHA), thrombin peak height and plasmin peak height were set to 1.0 nM, and thrombin potential and plasmin potential were set to 1.0 nM·min to be able to include these results in the analyses.
For each run of the NHA, normal pooled plasma (NPP) was included as a test control. NPP consisted of equal amounts of PPP from 20 healthy donors in the age range of 18 to 70 years, with an equal representation of men and women. Women who were included were not pregnant and did not use oral contraceptives. All NHA results were measured in duplicate.
2.5. Healthy individuals
In order to compare TG and PG parameters of hemophilia patients with those of healthy individuals, the NHA was also measured in plasma samples of a control population. This control population comprised 16 healthy men, with age ranging from 26 to 59 years, not using drugs known to affect coagulation. All included individuals gave written informed consent.
2.6. Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics, version 25. Continuous variables are reported as medians with interquartile range (IQR). Categorical variables are presented as counts and percentages.
NHA parameters were obtained as absolute values and were used to analyze differences in TG and PG parameters at baseline between patients with severe, moderate, or mild hemophilia and healthy individuals. The Kruskal-Wallis test and subsequently the Mann-Whitney U tests for post hoc analysis were used to classify these differences. P values of <.05 were considered statistically significant. A Bonferroni correction was applied to the post hoc Mann-Whitney U tests. The post hoc analysis was considered statistically significant at P < .0167.
To describe the association between TG and PG parameters and the clinical bleeding phenotype, NHA parameters were obtained as percentage of normal. The absolute values of the NHA parameters of the HiN6 cohort were divided by the medians of the NHA parameters of healthy individuals, which is endorsed by the ISTH guidelines on TGAs, to improve comparability of results between studies [26]. First, the associations between TG and PG parameters and the A(J)BR were assessed. For these analyses, all patients using prophylactic therapy were excluded to avoid that the associations are affected by prophylaxis. Second, differences in baseline NHA parameters between patients with a mild or a severe clinical bleeding phenotype were analyzed using the Mann-Whitney U test. Patients using primary prophylaxis were excluded from these analyses as they were not classified into having a mild or a severe clinical bleeding phenotype. Patients on secondary and tertiary prophylaxis were included in these analyses as they already have established their severe bleeding phenotype.
3. Results
3.1. Patient characteristics
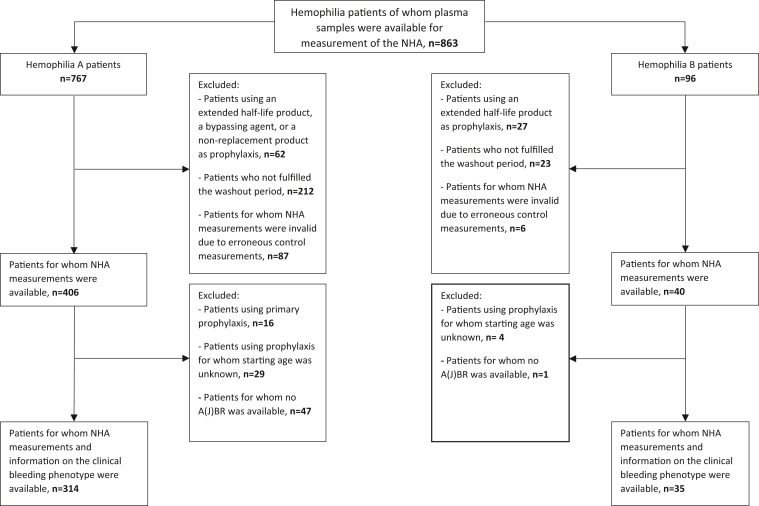
In total, 446 plasma samples of patients with hemophilia were included in this substudy. More detailed information is provided by the flow diagram in Figure 1. Patient characteristics are shown in the Table. The median age at the time of inclusion was 44 years (range: 0.4 to 86 years). In both the HA and HB patient group, the largest group included were patients with mild disease (63%). In the study population, 90% of the patients never had a neutralizing inhibitor against FVIII or FIX. A concomitant coagulation disorder was present in 2% of the patients, of which von Willebrand disease was the most prevalent.
Figure 1.
Flow diagram of patients included in the study.
Table.
Baseline characteristics of patients included in the study.
| Characteristics | Hemophilia A (n = 406) | Hemophilia B (n = 40) | Total group (n = 446) |
|---|---|---|---|
| Age (y), median (range) | 46 (0.4-86) | 40 (13-73) | 44 (0.4-86) |
| Age categories (%) | |||
| Children, 0-17 y | 48 (12) | 1 (3) | 49 (11) |
| Adults, ≥18 y | 358 (88) | 39 (98) | 397 (89) |
| Severity of hemophilia (%) | |||
| Severe | 67 (17) | 5 (13) | 72 (16) |
| Moderate | 82 (20) | 10 (25) | 92 (21) |
| Mild | 257 (63) | 25 (63) | 282 (63) |
| Patients using prophylaxis (%) | 56 (14) | 4 (10) | 60 (13) |
| Inhibitor status (%) | |||
| Never | 363 (89) | 40 (100) | 403 (90) |
| Past | 33 (8) | 33 (7) | |
| Current | 9 (2) | 9 (2) | |
| Unknown | 1 | 1 | |
| Concomitant coagulation disorder (%) | |||
| Von Willebrand disease | 8 (2)a | 0 | 8 (2)a |
| Thrombocytopathy | 1b | 0 | 1b |
| Clotting factor deficiency | 2c | 0 | 2c |
Data were missing for 1 patient.
Data were missing for 5 patients.
Data were missing for 2 patients.
3.2. Baseline values of thrombin and plasmin generation
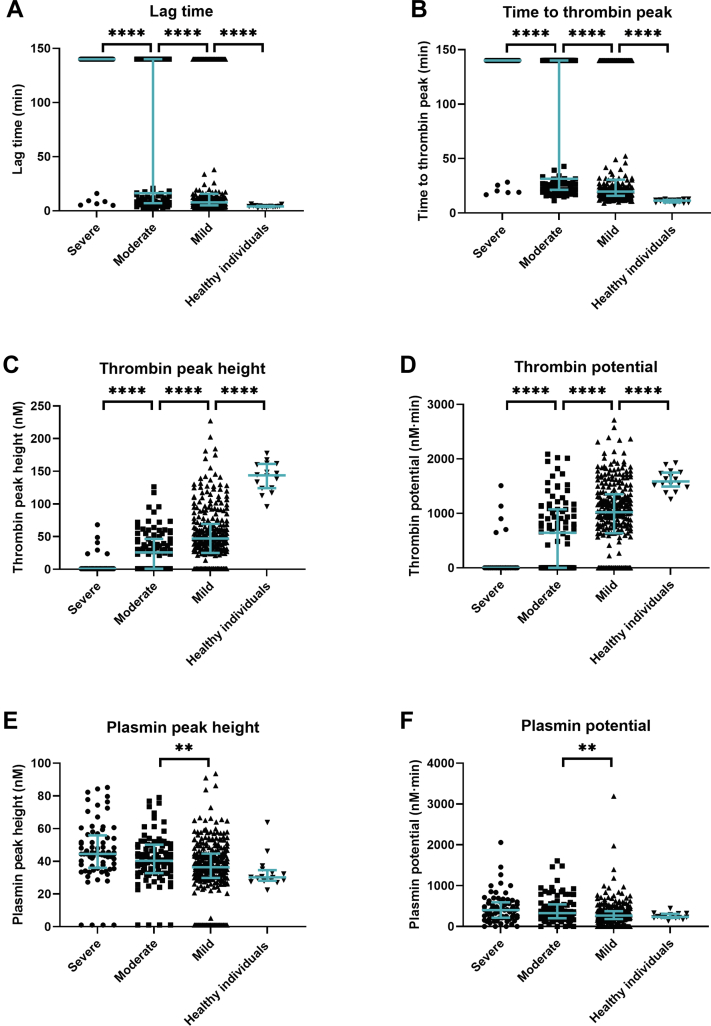
Results of baseline TG and PG parameters are shown in Figure 2. As the TG and PG parameters of patients with HA or HB did not significantly differ (Mann-Whitney U test, P > .05), results of both patient groups were combined. In patients with severe hemophilia, the lag time and time to thrombin peak were significantly longer than in patients with moderate or mild hemophilia (Figure 2A, B). In addition, the thrombin peak height and thrombin potential were significantly lower in patients with severe hemophilia (Figure 2C, D). The median thrombin peak height was 1.0 nM in patients with severe hemophilia, 25.9 nM in patients with moderate hemophilia, and 47.1 nM in patients with mild hemophilia. The median thrombin potentials in these patients were 1.0 nM·min, 644.7 nM·min, and 1021.0 nM·min, respectively. Furthermore, a large heterogeneity in thrombin peak height and thrombin potential was observed in both patients with moderate and mild hemophilia. All TG parameters significantly differed between patients with hemophilia and healthy individuals.
Figure 2.
Thrombin and plasmin generation parameters at baseline in patients with hemophilia compared to healthy individuals. Parameters given are lag time (A), time to thrombin peak (B), thrombin peak height (C), thrombin potential (D), plasmin peak height (E), and plasmin potential (F). Lines represent median with interquartile range in all graphs. ∗∗P < .01; ∗∗∗∗P < .0001.
Plasmin peak height and plasmin potential were increased in patients with severe hemophilia compared to patients with moderate or mild hemophilia (Figure 2E, F). Both parameters were higher in patients with hemophilia than in healthy individuals. For the FLT, no differences were observed between hemophilia severities and healthy individuals (Figure 1, Supplemenatry Material). For each TG and PG parameter, the median values and interquartile ranges are shown in the Supplementary Table.
3.3. Association between thrombin generation and clinical bleeding phenotype
For the assessment of the association between TG parameters and the A(J)BR, data of 318 adults (284 HA, 34 HB) and 20 children (19 HA, 1 HB) with hemophilia were included (Figure 1). As the bleeding phenotypes of patients with HA or B did not differ (chi-squared test, P > .05), again the results of both patient groups were combined.
3.3.1. Adults with hemophilia
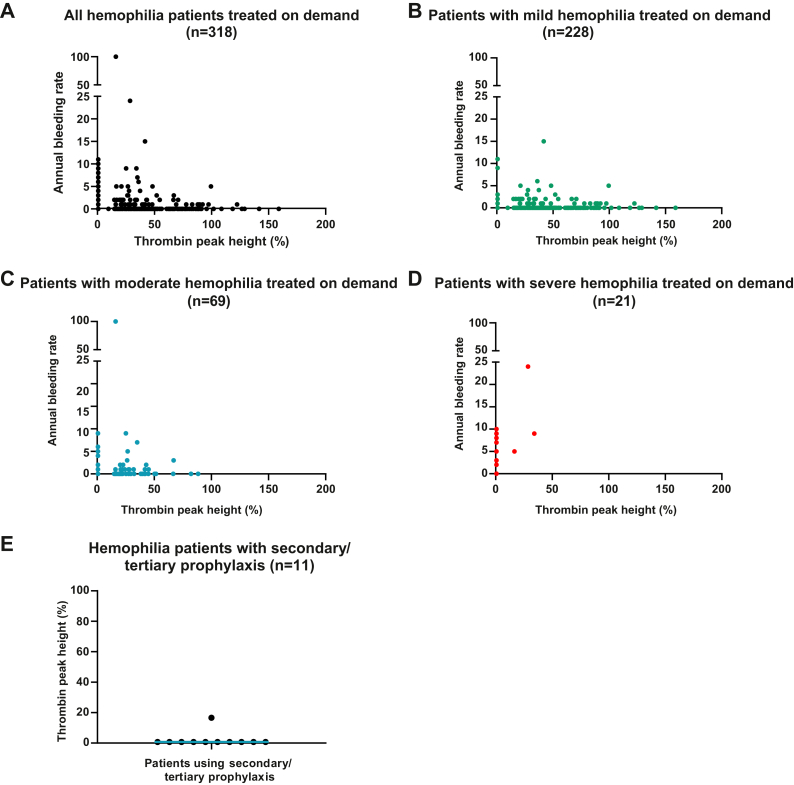
An inverse association was found between thrombin peak height and ABR (Figure 3A). The majority of patients with an ABR of ≥5 (severe bleeding phenotype) had a thrombin peak height of <49% compared to healthy individuals. Interestingly, such a decreased thrombin peak height in combination with an ABR of ≥5 (severe bleeding phenotype) was found in patients with mild (Figure 3B), moderate (Figure 3C), and severe hemophilia (Figure 3D). Furthermore, all patients receiving secondary/tertiary prophylaxis had a thrombin peak height of <17% (Figure 3E) after accomplishing the washout period.
Figure 3.
Association between annual bleeding rate and thrombin peak height in adult patients with hemophilia. (A) Annual bleeding rate according to thrombin peak height in all patients included with on-demand treatment (n = 318). This group includes patients with mild (n = 228), moderate (n = 69), and severe hemophilia (n = 21). (B) Annual bleeding rate according to thrombin peak height in patients with mild hemophilia treated on demand. (C) Annual bleeding rate according to thrombin peak height in patients with moderate hemophilia treated on demand. (D) Annual bleeding rate according to thrombin peak height in patients with severe hemophilia treated on demand. (E) Thrombin peak height in patients receiving secondary/tertiary prophylaxis (n = 11). Thrombin peak height is presented as the percentage of normal.
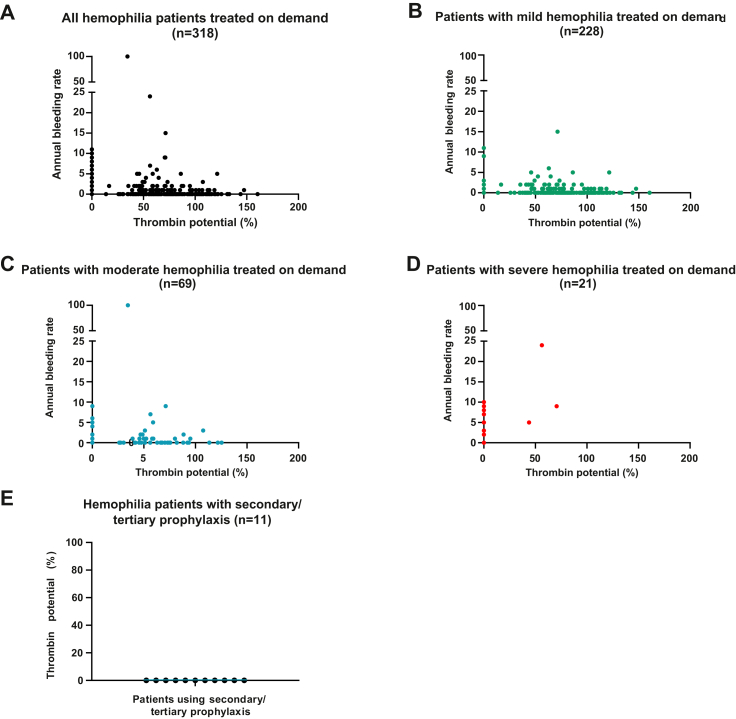
In addition, an inverse association was found between thrombin potential and ABR (Figure 4A). The majority of patients with an ABR of ≥5 (severe bleeding phenotype) had a thrombin potential of <72% compared to healthy individuals. Again, all hemophilia severity categories were represented with such a decreased thrombin potential in combination with an ABR of ≥5 (severe bleeding phenotype; Figure 4B–D). Off the patients receiving secondary/tertiary prophylaxis, all patients had a thrombin potential of <1% after accomplishing the washout period (Figure 4E).
Figure 4.
Associations between annual bleeding rate and thrombin potential in adult persons with hemophilia. (A) Annual bleeding rate according to thrombin potential in all patients included with on-demand treatment (n = 318). This group includes patients with mild (n = 228), moderate (n = 69), and severe hemophilia (n = 21). (B) Annual bleeding rate according to thrombin potential in patients with mild hemophilia treated on demand. (C) Annual bleeding rate according to thrombin potential in patients with moderate hemophilia treated on demand. (D) Annual bleeding rate according to thrombin potential in patients with severe hemophilia treated on demand. (E) Thrombin potential in patients receiving secondary/tertiary prophylaxis (n = 11). Thrombin potential is presented as the percentage of normal.
Furthermore, an inverse association was found between thrombin peak height and AJBR (Figure 2A, Supplementary Material) and thrombin potential and AJBR (Figure 3A, Supplementary Material). The majority of patients with an AJBR of ≥3 (severe bleeding phenotype) had a thrombin peak height of <42% and a thrombin potential of <72%. Again, this severe bleeding phenotype with a concomitant low TG profile was represented by patients with mild (Figures 2B and 3B, Supplementary Material), moderate (Figures 2C and 3C, Supplementary Material), and severe hemophilia (Figures 2D and 3D, Supplementary Material). In addition, positive associations were found between the lag time and A(J)BR and the time to thrombin peak and A(J)BR. In other words, patients with a severe clinical bleeding phenotype had a decreased thrombin peak height and thrombin potential along with a delayed lag time and time to thrombin peak compared to healthy individuals. PG parameters did not show an association with the clinical bleeding phenotype (data not shown).
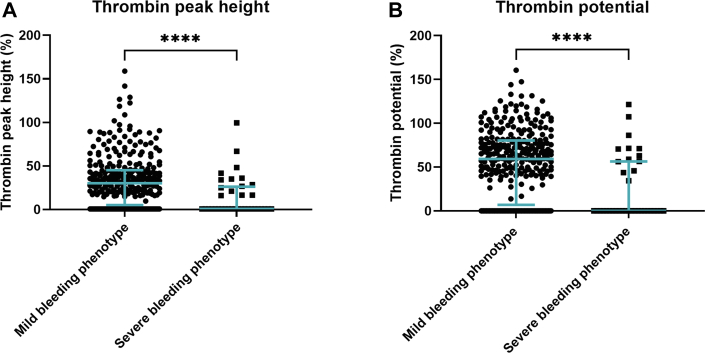
TG parameters of hemophilia patients with a mild or a severe clinical bleeding phenotype were compared (Figure 5). For these analyses, data of 329 (295 with HA, 34 with HB) adults were included. In patients with an ABR of <5 and an AJBR of <3 who did not receive prophylactic replacement therapy, the median thrombin peak height and median thrombin potential were 30.3% and 59.3%, respectively. In patients with an ABR of ≥5 and/or an AJBR of ≥3 and/or in patients receiving secondary/tertiary prophylaxis, the median thrombin peak height was 0.70% and the median thrombin potential was 0.06%. In brief, medians were significantly different between hemophilia patients with a mild or severe clinical bleeding phenotype.
Figure 5.
Thrombin peak height and thrombin potential according to clinical bleeding phenotype. (A) Thrombin peak height in hemophilia patients with a mild clinical bleeding phenotype (patients with an ABR of <5 and an AJBR of <3, and no prophylactic replacement therapy) or a severe clinical bleeding phenotype (patients with an ABR of ≥5 and/or an AJBR of ≥3 and/or those who receive secondary/tertiary prophylaxis). (B) Thrombin potential in patients with a mild clinical bleeding phenotype (patients with an ABR of <5 and an AJBR of <3, and no prophylactic replacement therapy) or a severe clinical bleeding phenotype (patients with an ABR of ≥5 and/or an AJBR of ≥3 and/or those who receive secondary/tertiary prophylaxis). NHA parameters are presented as the percentage of normal. Lines represent median with interquartile range. ∗∗∗∗P < .0001.
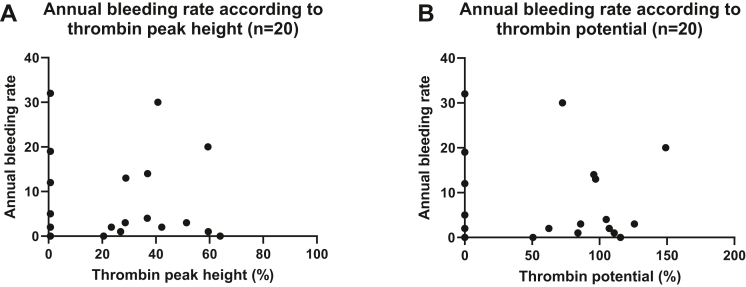
3.3.2. Children with hemophilia
No association was found between BR and thrombin peak height and BR (Figure 6A) and thrombin potential (Figure 6B) in children with hemophilia. All children reporting a bleeding episode had a thrombin peak height of <60%. No child reported a joint bleeding, and therefore, associations between JBR, thrombin peak height, and thrombin potential could not be obtained.
Figure 6.
Association between bleeding rate and thrombin peak height and thrombin potential in children with hemophilia. (A) Bleeding rate according to thrombin peak height in children with hemophilia treated on demand (n = 20). (B) Bleeding rate according to thrombin potential in children with hemophilia treated on demand (n = 20). NHA parameters are presented as the percentage of normal.
4. Discussion
In this large nationwide cross-sectional study in Dutch hemophilia patients, substantial differences in TG parameters were revealed between patients with severe, moderate, and mild hemophilia and healthy individuals. Taking into account the bleeding phenotype of these hemophilia patients, there was an obvious association between patients with a severe bleeding phenotype and a lower TG profile.
Overall, patients with severe hemophilia had the longest lag time and time to thrombin peak and the lowest thrombin peak height and thrombin potential. This is in accordance with previous studies, which reported significant differences in time to thrombin peak, thrombin peak height, and thrombin potential between patients with a different hemophilia severity [4,6,10]. However, a significant difference in lag time as described in our study has never been observed. This difference can be explained by the assay setup. The NHA initiates TG with a TF concentration of 0.28 pM [23], instead of a concentration of 1 pM or 5 pM, which is normally used to trigger TGAs. This makes the NHA more sensitive to small changes in lag time.
Patients with severe hemophilia showed a higher plasmin peak height and plasmin potential compared with patients with moderate or mild hemophilia or healthy individuals. This result is in line with previous studies, which reported an enhanced fibrinolytic capacity in patients with low TG. It is suggested that the hyperfibrinolytic state in these patients is caused by reduced activation of the thrombin activatable fibrinolysis inhibitor (TAFI) [10,21,22]. However, no association between the PG profile and the clinical bleeding phenotype was observed. A similar unexpected finding was reported by the study of van Dijk et al., which did not find a significant difference in clot lysis time between hemophilia patients with a mild or severe clinical bleeding phenotype [27].
Inverse associations were found between A(J)BR, thrombin peak height, and thrombin potential in adults with hemophilia. A severe clinical bleeding phenotype (patients with an ABR of ≥5 and/or an AJBR of ≥3 and/or patients receiving secondary/tertiary prophylaxis) was found in adults with a thrombin peak height of <49% or a thrombin potential of <72%, and was independent of hemophilia severity. However, 2 patients reported a severe clinical bleeding phenotype despite a thrombin peak height of 67% and 100%, respectively, and a thrombin potential of 107% and 121%, respectively. The first patient, with moderate hemophilia, had a negative inhibitor titer and did not use any anticoagulant drugs. This patient reported 3 joint bleedings. It is possible that his lifestyle (ie, his work, sport activities, hobbies, etc.) contributed to the occurrence of these bleedings. The second patient had mild hemophilia and a negative inhibitor titer, and did not use any anticoagulant medication. He only reported mucosal and wound bleedings, which are most likely trauma-related. Previously, the study of Dargaud et al. showed that trauma-related bleedings do not correlate with the TG profile [12]. Furthermore, this is probably the reason why no association was found between either BR and thrombin peak height or thrombin potential in children with hemophilia. Children mostly reported epistaxis, hematomas, and wound bleedings, which is consistent with the literature [28], and are most likely related with trauma. That no child reported a joint bleeding may be due to the fact that the JBR was calculated based on a period of 3 months, which is probably too short especially if they are very young.
Thrombin peak height and thrombin potential were significantly lower in patients with a severe clinical bleeding phenotype than in patients with a mild clinical bleeding phenotype. Overall, it is remarkable that there were patients reporting a mild clinical bleeding phenotype despite low TG. This phenomenon seems to be consistent with previous research that found patients with low thrombin potential and no spontaneous bleeding episodes during a period of 6 months [12]. Further studies on these patients are needed to explore the hemostatic potential of this group.
This study has a number of limitations. First, patients using prophylactic therapy were needed to be excluded for the assessment of the associations between TG parameters and the A(J)BR as prophylaxis affects the bleeding tendency. However, the exclusion of these patients may have led to the withdrawal of patients with the most severe clinical bleeding phenotype and therefore to an underestimation of the associations observed. Patients receiving prophylactic treatment, mostly those with severe hemophilia, are particularly expected to have the most severe bleeding tendency (if not on prophylaxis) and to have the lowest TG profile. For the same reason, exclusion of patients on primary prophylaxis for the analyses of the differences in TG parameters between patients with a mild or severe clinical bleeding phenotype may have led to a bias in our results. However, this problem will apply to every study on the clinical bleeding phenotype of patients with hemophilia in prosperous countries, as primary prophylaxis is the standard of care.
Second, information on bleeding episodes was patient-reported, whereas for our goals, it might have been better to categorize bleeding events with more detailed, prespecified objective criteria. The significance and severity of bleeding episodes were therefore unclear. Yet, patients were asked to only report bleeding episodes that required administration of DDAVP, FVIII, or FIX concentrates or bypassing agents during 2 or more days, thereby reducing the bias that might have occurred if patients would have reported also other, less severe, bleeding episodes.
Third, information on the cause of a bleeding episode (ie, trauma-related, spontaneous, or postoperatively) was missing, while a previous study by Dargaud et al. showed that bleedings of any cause do not correlate with the TG profile [12]. Therefore, there is a possibility that in this study, the associations between the clinical bleeding phenotype and TG parameters are underestimated. However, the purpose of this study was to show that a severe clinical bleeding phenotype may exist in all kinds of hemophilia patients, even those with mild hemophilia. For the entire group of hemophilia patients with a severe bleeding phenotype, the TG profile was low. However, for the individual patient, it is still a challenge to translate this low TG into a precise risk of spontaneous bleeding.
Fourth, information on the washout period of patients on prophylactic replacement therapy was patient-reported. Three patients with severe hemophilia and 1 patient with moderate hemophilia did not report FVIII or FIX administration in the last 72 hours or 120 hours, but showed TG profiles that suggested otherwise. In these patients, the FVIII or FIX activity level was higher than expected based on the hemophilia severity of the patient. This suggests that patients did not always report the washout period correctly. Other explanations could be that these patients metabolize the factor concentrates more slowly than average or have a relatively high von Willebrand activity.
A fifth limitation that needs to be mentioned applies to every study using a TGA. Results are difficult to generalize due to the lack of standardization protocols (ie, TF concentration, use of corn trypsin inhibitor, PPP, or platelet rich plasma). However, as is formulated by the ISTH in guidelines for TG assays [26], in our study, the results of patients were compared with those of healthy individuals and presented as the percentage of normal.
In the future, a large study with more granular, objective information on bleeding episodes is needed. For instance, information about the cause of a bleeding should be specified (ie, spontaneous, trauma-related, or postoperative). In addition, information on the age of first (joint) bleed, joint damage, and annual factor consumption should be obtained in order to better quantify the association between the TG profile and the clinical bleeding phenotype. Furthermore, additional research is needed to assess the influence of ethnicity, also looking into the differences in genotype and immune status. However, based on our study, the association between TG profile and bleeding phenotype can potentially be used to better predict which patient requires early or more intensified prophylactic replacement therapy. Especially in the current era of new expensive treatment modalities such as emicizumab, anti-TF pathway inhibitors, antithrombin inhibitors, and gene therapy, this is even more important. It would be of great clinical value to decide upon an individual TG profile and bleeding tendency which treatment modality would be the most suitable to correct hemostasis instead of providing concentrates and nonfactor agents solely based on severe FVIII or FIX activity levels.
In conclusion, results from this study show that a severe clinical bleeding phenotype is associated with a decreased TG profile, and is independent of hemophilia severity based on factor activity levels. Which patients require early prophylactic replacement therapy is now based on the FVIII or FIX activity level. However, the TG profile of patients in combination with the clinical bleeding severity might harbor additional informative data of an individual hemostatic balance.
Acknowledgments
The authors would like to thank Wideke Barteling and Kitty Knobbe-Verbeek for the NHA measurements. This work was performed using the Parelsnoer Clinical Biobanks at Health-RI.
Funding
The Hemophilia in the Netherlands study was funded by the Dutch Ministry of Health, Welfare and Sports as part of the Hemophilia in the Netherlands study and by the Dutch Hemophilia Foundation (Stichting Haemophilia). Bayer provided an unrestricted research grant to conduct this substudy.
Author contributions
J.G.v.d.B., E.A.M.B., M.C., S.C.G., F.W.G.L., L.F.D.v.V., and S.E.M.S. are members of the steering committee that designed the study and performed data collection. M.J.A.V. performed statistical analysis, interpreted data, and wrote the manuscript. W.L.v.H., D.M., and S.E.M.S. interpreted data. S.E.M.S. wrote the manuscript. All authors critically revised and approved the final version of the paper.
Relationship Disclosure
M.J.A.V., E.A.M.B., N.M.A.B., J.H.J., and D.M. have no conflicts of interest to declare. W.L.V. received unrestricted grants from Bayer, Shire, Novo Nordisk, and CSL Behring, and is the founder and CSO of Enzyre BV, a Radboudumc spinoff company. J.G.V. has received unrestricted grants from Novo Nordisk and has been a teacher of the educational activities of Bayer. M.C. has received financial support for research from Bayer, CSL Behring, Daiichi Sankyo, Portola/Alexion, Roche, Sanquin Blood Supply, and uniQure and consultancy or lecturing fees from Bayer, CSL Behring, Medcon International, Medtalks, Novo Nordisk, Pfizer, and Sobi. S.C.G. has received unrestricted research grants from Sobi. F.W.G.L. has received unrestricted grants or research funding from CSL Behring, Sobi, Takeda, and uniQure and consultancy fees from BioMarin, CSL Behring, Takeda, and uniQure (of which all fees go to the university), and was a Data Safety Monitoring Board Member of Roche. L.F.D.V. has received a research grant from CSL Behring, and is a consultant for Sobi and Tremeau (all fees go to the institution). S.E.M.S. received an unrestricted research grant from Bayer.
Data availability
Data including study protocol and informed consent form are available upon request from the project coordinator S.C.G. (email: s.c.gouw@lumc.nl). Individual participant data will be made available after de-identification.
Footnotes
Funding information Dutch Ministry of Health, Welfare and Sports
Dutch Hemophilia Foundation (Stichting Haemophilia)
Bayer
Handling Editor: Dr Vania Morelli
The online version contains supplementary material available at https://doi.org/10.1016/j.rpth.2023.100062
Supporting Information
References
- 1.Srivastava A, Santagostino E, Dougall A, Kitchen S, Sutherland M, Pipe SW, et al. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia 2020;26:1–158. [DOI] [PubMed]
- 2.Jayandharan G.R., Srivastava A. The phenotypic heterogeneity of severe hemophilia. Semin Thromb Hemost. 2008;34:128–141. doi: 10.1055/s-2008-1066024. [DOI] [PubMed] [Google Scholar]
- 3.Beltrán-Miranda C.P., Khan A., Jaloma-Cruz A.R., Laffan M.A. Thrombin generation and phenotypic correlation in haemophilia A. Haemophilia. 2005;11:326–334. doi: 10.1111/j.1365-2516.2005.01107.x. [DOI] [PubMed] [Google Scholar]
- 4.Dargaud Y., Béguin S., Lienhart A., Al Dieri R., Trzeciak C., Bordet J.C., et al. Evaluation of thrombin generating capacity in plasma from patients with haemophilia A and B. Thromb Haemost. 2005;93:475–480. doi: 10.1160/TH04-10-0706. [DOI] [PubMed] [Google Scholar]
- 5.Lewis S.J., Stephens E., Florou G., Macartney N.J., Hathaway L.S., Knipping J., et al. Measurement of global haemostasis in severe haemophilia A following factor VIII infusion. Br J Haematol. 2007;138:775–782. doi: 10.1111/j.1365-2141.2007.06722.x. [DOI] [PubMed] [Google Scholar]
- 6.van Veen J.J., Gatt A., Bowyer A.E., Cooper P.C., Kitchen S., Makris M. Calibrated automated thrombin generation and modified thromboelastometry in haemophilia A. Thromb Res. 2009;123:895–901. doi: 10.1016/j.thromres.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Ay Y., Balkan C., Karapinar D.Y., Akin M., Bilenoğlu B., Kavakli K. Feasibility of using thrombin generation assay (TGA) for monitoring of haemostasis during supplementation therapy in haemophilic patients without inhibitors. Haemophilia. 2012;18:911–916. doi: 10.1111/j.1365-2516.2012.02849.x. [DOI] [PubMed] [Google Scholar]
- 8.Al Hawaj M.A., Martin E.J., Venitz J., Barrett J.C., Kuhn J.G., Nolte M.E., et al. Monitoring rFVIII prophylaxis dosing using global haemostasis assays. Haemophilia. 2013;19:409–414. doi: 10.1111/hae.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chelle P., Montmartin A., Piot M., Ardillon L., Wibaut B., Frotscher B., et al. Prediction of individual factor VIII or IX level for the correction of thrombin generation in haemophilic patients. Haemophilia. 2018;24:995–1001. doi: 10.1111/hae.13539. [DOI] [PubMed] [Google Scholar]
- 10.Valke L.L.F.G., Bukkems L.H., Barteling W., Laros-van Gorkom B.A.P., Blijlevens N.M.A., Mathôt R.A.A., et al. Pharmacodynamic monitoring of factor VIII replacement therapy in hemophilia A: combining thrombin and plasmin generation. J Thromb Haemost. 2020;18:3222–3231. doi: 10.1111/jth.15106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brummel-Ziedins K.E., Whelihan M.F., Gissel M., Mann K.G., Rivard G.E. Thrombin generation and bleeding in haemophilia A. Haemophilia. 2009;15:1118–1125. doi: 10.1111/j.1365-2516.2009.01994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dargaud Y., Negrier C., Rusen L., Windyga J., Georgiev P., Bichler J., et al. Individual thrombin generation and spontaneous bleeding rate during personalized prophylaxis with Nuwiq® (human-cl rhFVIII) in previously treated patients with severe haemophilia A. Haemophilia. 2018;24:619–627. doi: 10.1111/hae.13493. [DOI] [PubMed] [Google Scholar]
- 13.Dave R.G., Geevar T., Mammen J.J., Vijayan R., Mahasampath G., Nair S.C. Clinical utility of activated partial thromboplastin time clot waveform analysis and thrombin generation test in the evaluation of bleeding phenotype in hemophilia A. Indian J Pathol Microbiol. 2021;64:117–122. doi: 10.4103/IJPM.IJPM_336_19. [DOI] [PubMed] [Google Scholar]
- 14.Måseide R.J., Berntorp E., Nummi V., Lassila R., Tjønnfjord G.E., Holme P.A. Bleeding phenotype of patients with moderate haemophilia A and B assessed by thromboelastometry and thrombin generation. Haemophilia. 2021;27:793–801. doi: 10.1111/hae.14355. [DOI] [PubMed] [Google Scholar]
- 15.Milos M., Coen Herak D., Mahmoud Hourani Soutari N., Pavic J., Zupancic-Salek S., Zadro R., et al. Overall hemostasis potential and aPTT-clot waveform analysis as powerful laboratory diagnostic tools for identification of hemophilia A patients with unexpected bleeding phenotype. Int J Lab Hematol. 2021;43:273–280. doi: 10.1111/ijlh.13347. [DOI] [PubMed] [Google Scholar]
- 16.Santagostino E., Mancuso M.E., Tripodi A., Chantarangkul V., Clerici M., Garagiola I., et al. Severe hemophilia with mild bleeding phenotype: molecular characterization and global coagulation profile. J Thromb Haemost. 2010;8:737–743. doi: 10.1111/j.1538-7836.2010.03767.x. [DOI] [PubMed] [Google Scholar]
- 17.Tarandovskiy I.D., Balandina A.N., Kopylov K.G., Konyashina N.I., Kumskova M.A., Panteleev M.A., et al. Investigation of the phenotype heterogeneity in severe hemophilia A using thromboelastography, thrombin generation, and thrombodynamics. Thromb Res. 2013;131:e274–e280. doi: 10.1016/j.thromres.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Delavenne X., Ollier E., Lienhart A., Dargaud Y. A new paradigm for personalized prophylaxis for patients with severe haemophilia A. Haemophilia. 2020;26:228–235. doi: 10.1111/hae.13935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bukkems L.H., Valke L.L.F.G., Barteling W., Laros-van Gorkom B.A.P., Blijlevens N.M.A., Cnossen M.H., et al. Combining factor VIII levels and thrombin/plasmin generation: a population pharmacokinetic-pharmacodynamic model for patients with haemophilia A. Br J Clin Pharmacol. 2022;88:2757–2768. doi: 10.1111/bcp.15185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verhagen M.J.A., Valke L.L.F.G., Schols S.E.M. Thrombin generation for monitoring hemostatic therapy in hemophilia A: a narrative review. J Thromb Haemost. 2022;20:P794–P805. doi: 10.1111/jth.15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broze G.J., Jr., Higuchi D.A. Coagulation-dependent inhibition of fibrinolysis: role of carboxypeptidase-U and the premature lysis of clots from hemophilic plasma. Blood. 1996;88:3815–3823. [PubMed] [Google Scholar]
- 22.Mosnier L.O., Lisman T., van den Berg H.M., Nieuwenhuis H.K., Meijers J.C., Bouma B.N. The defective down regulation of fibrinolysis in haemophilia A can be restored by increasing the TAFI plasma concentration. Thromb Haemost. 2001;86:1035–1039. [PubMed] [Google Scholar]
- 23.van Geffen M., Loof A., Lap P., Boezeman J., Laros-van Gorkom B.A., Brons P., et al. A novel hemostasis assay for the simultaneous measurement of coagulation and fibrinolysis. Hematology. 2011;16:327–336. doi: 10.1179/102453311X13085644680348. [DOI] [PubMed] [Google Scholar]
- 24.Hassan S., van Balen E.C., Smit C., Mauser-Bunschoten E.P., van Vulpen L.F.D., Eikenboom J., et al. Health and treatment outcomes of patients with hemophilia in the Netherlands, 1972-2019. J Thromb Haemost. 2021;19:2394–2406. doi: 10.1111/jth.15424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saes J.L., Schols S.E.M., Betbadal K.F., van Geffen M., Verbeek-Knobbe K., Gupta S., et al. Thrombin and plasmin generation in patients with plasminogen or plasminogen activator inhibitor type 1 deficiency. Haemophilia. 2019;25:1073–1082. doi: 10.1111/hae.13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dargaud Y., Wolberg A.S., Gray E., Negrier C., Hemker H.C. Subcommittee on Factor VIII, Factor IX, and Rare Coagulation Disorders. Proposal for standardized preanalytical and analytical conditions for measuring thrombin generation in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2017;15:1704–1707. doi: 10.1111/jth.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Dijk K., van der Bom J.G., Fischer K., de Groot P.G., van den Berg H.M. Phenotype of severe hemophilia A and plasma levels of risk factors for thrombosis. J Thromb Haemost. 2007;5:1062–1064. doi: 10.1111/j.1538-7836.2007.02447.x. [DOI] [PubMed] [Google Scholar]
- 28.Ragni M.V., Fogarty P.J., Josephson N.C., Neff A.T., Raffini L.J., Kessler C.M. Survey of current prophylaxis practices and bleeding characteristics of children with severe haemophilia A in US haemophilia treatment centres. Haemophilia. 2012;18:63–68. doi: 10.1111/j.1365-2516.2011.02554.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data including study protocol and informed consent form are available upon request from the project coordinator S.C.G. (email: s.c.gouw@lumc.nl). Individual participant data will be made available after de-identification.