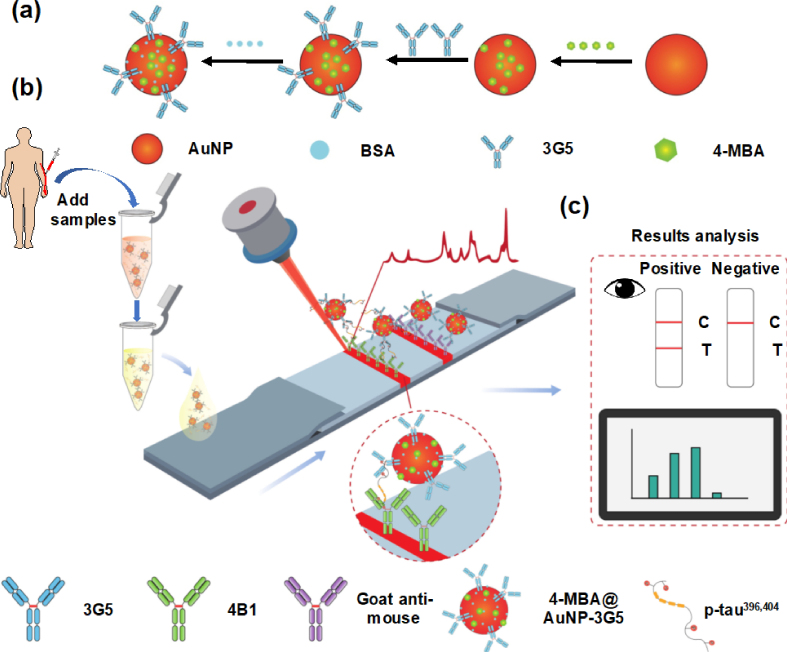
Abstract
Phosphorylation of tau at Ser (396, 404) (p-tau396,404) is one of the earliest phosphorylation events, and plasma p-tau396,404 level appears to be a potentially promising biomarker of Alzheimer’s disease (AD). The low abundance and easy degradation of p-tau in the plasma make the lateral flow assay (LFA) a suitable choice for point-of-care detection of plasma p-tau396,404 levels. Herein, based on our screening of a pair of p-tau396,404-specific antibodies, we developed a colorimetric and surface-enhanced Raman scattering (SERS) dual-readout LFA for the rapid, highly sensitive, and robust detection of plasma p-tau396,404 levels. This LFA realized a detection limit of 60 pg/mL by the naked eye or 3.8 pg/mL by SERS without cross-reacting with other tau species. More importantly, LFA rapidly and accurately differentiated AD patients from healthy controls, suggesting that it has the potential for clinical point-of-care application in AD diagnosis. This dual-readout LFA has the advantages of simple operation, rapid, and ultra-sensitive detection, providing a new way for early AD diagnosis and intervention, especially in primary and community AD screening.
Electronic Supplementary Material
Supplementary material (characterization of AuNPs and 4-MBA@AuNP probe; the optimal 4-MBA load for AuNPs; the optimal K2CO3 volumes for 4-MBA@AuNP-3G5 conjugates; the optimal 3G5 load for 4-MBA@AuNP conjugates; effect of NaCl concentration on 4-MBA@AuNP-3G5 stability; the linear curve of T-line color and SERS intensity versus different p-tau396,404 concentrations; the comparison of colorimetric-based LFA test results and the diagnosis results; Raman intensities and antibody activity of 4-MBA@AuNP-3G5 before and after storage; colorimetric intensity of dual-readout LFA detecting different concentrations of p-tau396,404 protein; sequence of synthesized peptides used in this study; information of the participants in this study; the information of antibodies used in this study) is available in the online version of this article at 10.1007/s12274-022-5354-4.
Keywords: Alzheimer’s disease; p-tau396,404; plasma detection; surface-enhanced Raman scattering; lateral flow assay
Electronic Supplementary Material
Ultrasensitive and point-of-care detection of plasma phosphorylated tau in Alzheimer’s disease using colorimetric and surface-enhanced Raman scattering dual-readout lateral flow assay
Acknowledgements
The authors would like to thank the technical support from Xuewei Du of the China University of Geosciences and Associate Professor Jinyang Zhang of Kunming University of Science and Technology in the screening of monoclonal antibodies. This study was financially supported by the National Science and Technology Innovation 2030 (Nos. 2021ZD0201000 and 2021ZD0201001), the National Natural Science Foundation of China (No. 81971025), and the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (No. 2019-I2M-5-014). We also thank the Optical Bioimaging Core Facility and the Center for Nanoscale Characterization & Devices (CNCD) of WNLO-HUST, the Analytical and Testing Center of HUST, and the Research Core Facilities for Life Science (HUST) for support with data acquisition. We thank all patients and healthy individuals for donating their blood samples.
Footnotes
Liding Zhang and Ying Su contributed equally to this work.
References
- [1].McDade E, Bateman R J. Stop Alzheimer’s before it starts. Nature. 2017;547:153–155. doi: 10.1038/547153a. [DOI] [PubMed] [Google Scholar]
- [2].Lane C A, Hardy J, Schott J M. Alzheimer’s disease. Eur. J. Neurol. 2018;25:59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- [3].Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen C E, Cummings J, van der Flier W M. Alzheimer’s disease. Lancet. 2021;397:1577–1590. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].GBD 2016 Dementia Collaborators Global, regional, and national burden of Alzheimer’s disease and other dementias. 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ittner L M, Götz J. Amyloid-β and tau—A toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci. 2011;12:65–72. doi: 10.1038/nrn2967. [DOI] [PubMed] [Google Scholar]
- [6].Hardy J A, Higgins G A. Alzheimer’s disease: The amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- [7].Giacobini E, Gold G. Alzheimer disease therapy-moving from amyloid-β to tau. Nat. Rev. Neurol. 2013;9:677–686. doi: 10.1038/nrneurol.2013.223. [DOI] [PubMed] [Google Scholar]
- [8].Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer’s disease. Lancet. 2011;377:1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- [9].Brazaca L C, Moreto J R, Martín A, Tehrani F, Wang J, Zucolotto V. Colorimetric paper-based immunosensor for simultaneous determination of fetuin B and clusterin toward early Alzheimer’s diagnosis. ACS Nano. 2019;13:13325–13332. doi: 10.1021/acsnano.9b06571. [DOI] [PubMed] [Google Scholar]
- [10].Zhang L D, Liang X H, Zhang Z H, Luo H M. Cerebrospinal fluid and blood biomarkers in the diagnostic assays of Alzheimer’s disease. J. Innov. Opt. Health Sci. 2022;15:2230001. [Google Scholar]
- [11].Hu S, Yang C W, Li Y Q, Luo Q M, Luo H M. Nanozyme sensor array based on manganese dioxide for the distinction between multiple amyloid β peptides and their dynamic aggregation process. Biosens. Bioelectron. 2022;199:113881. doi: 10.1016/j.bios.2021.113881. [DOI] [PubMed] [Google Scholar]
- [12].Mondragón-Rodríguez S, Perry G, Luna-Muñoz J, Acevedo-Aquino M C, Williams S. Phosphorylation of tau protein at sites Ser396-404 is one of the earliest events in Alzheimer’s disease and Down syndrome. Neuropathol. Appl. Neurobiol. 2014;40:121–135. doi: 10.1111/nan.12084. [DOI] [PubMed] [Google Scholar]
- [13].Gomes L A, Uytterhoeven V, Lopez-Sanmartin D, Tomé S O, Tousseyn T, Vandenberghe R, Vandenbulcke M, von Arnim C A F, Verstreken P, Thal D R. Maturation of neuronal AD-tau pathology involves site-specific phosphorylation of cytoplasmic and synaptic tau preceding conformational change and fibril formation. Acta Neuropathol. 2021;141:173–192. doi: 10.1007/s00401-020-02251-6. [DOI] [PubMed] [Google Scholar]
- [14].Ma H, Liu S L, Liu Y W, Zhu J Y, Han X X, Ozaki Y, Zhao B. In-situ fingerprinting phosphorylated proteins via surface-enhanced Raman spectroscopy: Single-site discrimination of Tau biomarkers in Alzheimer’s disease. Biosens. Bioelectron. 2021;171:112748. doi: 10.1016/j.bios.2020.112748. [DOI] [PubMed] [Google Scholar]
- [15].Shi Y C, Gu L H, Wang Q, Gao L J, Zhu J L, Lu X, Zhou F F, Zhu D, Zhang H S, Xie C M, et al. Platelet amyloid-β protein precursor (AβPP) ratio and phosphorylated tau as promising indicators for early Alzheimer’s disease. J. Gerontol. Ser. A. 2020;75:664–670. doi: 10.1093/gerona/glz005. [DOI] [PubMed] [Google Scholar]
- [16].Fiandaca M S, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz J B, Abner E L, Petersen R C, Federoff H J, Miller B L, et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement. 2015;11:600–607.e1. doi: 10.1016/j.jalz.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhang L, Cao K, Su Y, Hu S, Liang X, Luo Q, Luo H. Colorimetric and surface-enhanced Raman scattering dual-mode magnetic immunosensor for ultrasensitive detection of blood phosphorylated tau in Alzheimer’s disease. Biosens Bioelectron. 2023;222:114935. doi: 10.1016/j.bios.2022.114935. [DOI] [PubMed] [Google Scholar]
- [18].Ashton N J, Pascoal T A, Karikari T K, Benedet A L, Lantero-Rodriguez J, Brinkmalm G, Snellman A, Schöll M, Troakes C, Hye A, et al. Plasma p-tau231: A new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. 2021;141:709–724. doi: 10.1007/s00401-021-02275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Janelidze S, Mattsson N, Palmqvist S, Smith R, Beach T G, Serrano G E, Chai X Y, Proctor N K, Eichenlaub U, Zetterberg H, et al. Plasma P-tau181 in Alzheimer’s disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 2020;26:379–386. doi: 10.1038/s41591-020-0755-1. [DOI] [PubMed] [Google Scholar]
- [20].Thambisetty M, Lovestone S. Blood-based biomarkers of Alzheimer’s disease: Challenging but feasible. Biomark. Med. 2010;4:65–79. doi: 10.2217/bmm.09.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hampel H, O’Bryant S E, Molinuevo J L, Zetterberg H, Masters C L, Lista S, Kiddle S J, Batrla R, Blennow K. Blood-based biomarkers for Alzheimer disease: Mapping the road to the clinic. Nat. Rev. Neurol. 2018;14:639–652. doi: 10.1038/s41582-018-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Foley A R, Roseman G P, Chan K, Smart A, Finn T S, Yang K, Lokey R S, Millhauser G L, Raskatov J A. Evidence for aggregation-independent, PrPC-mediated Aβ cellular internalization. Proc. Natl. Acad. Sci. USA. 2020;117:28625–28631. doi: 10.1073/pnas.2009238117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cintron A F, Dalal N V, Dooyema J, Betarbet R, Walker L C. Transport of cargo from periphery to brain by circulating monocytes. Brain Res. 2015;1622:328–338. doi: 10.1016/j.brainres.2015.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Boluda S, Iba M, Zhang B, Raible K M, Lee V M Y, Trojanowski J Q. Differential induction and spread of tau pathology in young PS19 tau transgenic mice following intracerebral injections of pathological tau from Alzheimer’s disease or corticobasal degeneration brains. Acta Neuropathol. 2015;129:221–237. doi: 10.1007/s00401-014-1373-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Clavaguera F, Grueninger F, Tolnay M. Intercellular transfer of tau aggregates and spreading of tau pathology: Implications for therapeutic strategies. Neuropharmacology. 2014;76:9–15. doi: 10.1016/j.neuropharm.2013.08.037. [DOI] [PubMed] [Google Scholar]
- [26].Guo J L, Lee V M Y. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat. Med. 2014;20:130–138. doi: 10.1038/nm.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ye L, Hamaguchi T, Fritschi S K, Eisele Y S, Obermüller U, Jucker M, Walker L C. Progression of seed-induced Aβ deposition within the limbic connectome. Brain Pathol. 2015;25:743–752. doi: 10.1111/bpa.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lakshmi S, Essa M M, Hartman R E, Guillemin G J, Sivan S, Elumalai P. Exosomes in Alzheimer’s disease: Potential role as pathological mediators, biomarkers and therapeutic targets. Neurochem. Res. 2020;45:2553–2559. doi: 10.1007/s11064-020-03111-1. [DOI] [PubMed] [Google Scholar]
- [29].Jia L F, Qiu Q Q, Zhang H, Chu L, Du Y F, Zhang J W, Zhou C K, Liang F R, Shi S L, Wang S, et al. Concordance between the assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimers Dement. 2019;15:1071–1080. doi: 10.1016/j.jalz.2019.05.002. [DOI] [PubMed] [Google Scholar]
- [30].Barthélemy N R, Horie K, Sato C, Bateman R J. Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer’s disease. J. Exp. Med. 2020;217:e20200861. doi: 10.1084/jem.20200861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Thijssen E H, La Joie R, Wolf A, Strom A, Wang P, Iaccarino L, Bourakova V, Cobigo Y, Heuer H, Spina S, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med. 2020;26:387–397. doi: 10.1038/s41591-020-0762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hanger D P, Anderton B H, Noble W. Tau phosphorylation: The therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 2009;15:112–119. doi: 10.1016/j.molmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- [33].Liu L, Kwak H, Lawton T L, Jin S X, Meunier A L, Dang Y F, Ostaszewski B, Pietras A C, Stern A M, Selkoe D J. An ultra-sensitive immunoassay detects and quantifies soluble Aβ oligomers in human plasma. Alzheimers Dement. 2022;18:1186–1202. doi: 10.1002/alz.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Palmqvist S, Janelidze S, Quiroz Y T, Zetterberg H, Lopera F, Stomrud E, Su Y, Chen Y H, Serrano G E, Leuzy A, et al. Discriminative accuracy of plasma phospho-tau217 for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2020;324:772–781. doi: 10.1001/jama.2020.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu Y L, Zhan L, Qin Z P, Sackrison J, Bischof J C. Ultrasensitive and highly specific lateral flow assays for point-of-care diagnosis. ACS Nano. 2021;15:3593–3611. doi: 10.1021/acsnano.0c10035. [DOI] [PubMed] [Google Scholar]
- [36].Ren W, Mohammed S I, Wereley S, Irudayaraj J. Magnetic focus lateral flow sensor for detection of cervical cancer biomarkers. Anal. Chem. 2019;91:2876–2884. doi: 10.1021/acs.analchem.8b04848. [DOI] [PubMed] [Google Scholar]
- [37].Ruppert C, Kaiser L, Jacob L J, Laufer S, Kohl M, Deigner H P. Duplex Shiny app quantification of the sepsis biomarkers C-reactive protein and interleukin-6 in a fast quantum dot labeled lateral flow assay. J. Nanobiotechnol. 2020;18:130. doi: 10.1186/s12951-020-00688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kim H M, Kim J, An J, Bock S, Pham X H, Huynh K H, Choi Y, Hahm E, Song H, Kim J W, et al. Au-Ag assembled on silica nanoprobes for visual semiquantitative detection of prostate-specific antigen. J. Nanobiotechnol. 2021;19:73. doi: 10.1186/s12951-021-00817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Liu Q Y, Cheng S M, Chen R, Ke J M, Liu Y W, Li Y F, Feng W, Li F Y. Near-infrared lanthanide-doped nanoparticles for a low interference lateral flow immunoassay test. ACS Appl. Mater. Interfaces. 2020;12:4358–4365. doi: 10.1021/acsami.9b22449. [DOI] [PubMed] [Google Scholar]
- [40].Ji T X, Xu X Q, Wang X D, Cao N, Han X R, Wang M H, Chen B, Lin Z, Jia H Y, Deng M, et al. Background-free chromatographic detection of sepsis biomarker in clinical human serum through near-infrared to near-infrared upconversion immunolabeling. ACS Nano. 2020;14:16864–16874. doi: 10.1021/acsnano.0c05700. [DOI] [PubMed] [Google Scholar]
- [41].Xu K C, Zhou R, Takei K, Hong M H. Toward flexible surface-enhanced Raman scattering (SERS) sensors for point-of-care diagnostics. Adv. Sci. (Weinh.) 2019;6:1900925. doi: 10.1002/advs.201900925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Madzharova F, Heiner Z, Kneipp J. Surface enhanced hyper Raman scattering (SEHRS) and its applications. Chem. Soc. Rev. 2017;46:3980–3999. doi: 10.1039/c7cs00137a. [DOI] [PubMed] [Google Scholar]
- [43].Xia C, Zhang D, Li H, Li S, Liu H, Ding L, Liu X, Lyu M, Li R, Yang J, et al. Single-walled carbon nanotube based SERS substrate with single molecule sensitivity. Nano Research. 2022;15:694–700. [Google Scholar]
- [44].Shi L L, Xu L, Xiao R H, Zhou Z, Wang C W, Wang S Q, Gu B. Rapid, quantitative. high-sensitive detection of Escherichia coli O157: H7 by gold-shell silica-core nanospheres-based surface-enhanced Raman scattering lateral flow immunoassay. Front. Microbiol. 2020;11:596005. doi: 10.3389/fmicb.2020.596005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fu X L, Wen J H, Li J W, Lin H, Liu Y M, Zhuang X M, Tian C Y, Chen L X. Highly sensitive detection of prostate cancer specific PCA3 mimic DNA using SERS-based competitive lateral flow assay. Nanoscale. 2019;11:15530–15536. doi: 10.1039/c9nr04864b. [DOI] [PubMed] [Google Scholar]
- [46].Tian M, Wang J, Li C, Wang Z, Liu G, Lv E, Zhao X, Li Z, Cao D, Liu H, et al. Qualitative and quantitative detection of microcystin-LR based on SERS-FET dual-mode biosensor. Biosens Bioelectron. 2022;212:114434. doi: 10.1016/j.bios.2022.114434. [DOI] [PubMed] [Google Scholar]
- [47].Kim K, Han D K, Choi N, Kim S H, Joung Y, Kim K, Ho N T, Joo S W, Choo J. Surface-enhanced Raman scattering-based dual-flow lateral flow assay sensor for the ultrasensitive detection of the thyroid-stimulating hormone. Anal. Chem. 2021;93:6673–6681. doi: 10.1021/acs.analchem.0c05336. [DOI] [PubMed] [Google Scholar]
- [48].Cheng N, Lou B, Wang H. An intelligent serological SERS test toward early-stage hepatocellular carcinoma diagnosis through ultrasensitive nanobiosensing. Nano Research. 2022;15:5331–5339. [Google Scholar]
- [49].Liang J J, Teng P J, Xiao W, He G B, Song Q F, Zhang Y, Peng B, Li G, Hu L S, Cao D L, et al. Application of the amplification-free SERS-based CRISPR/Cas12a platform in the identification of SARS-CoV-2 from clinical samples. J. Nanobiotechnol. 2021;19:273. doi: 10.1186/s12951-021-01021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Perrault S D, Chan W C W. Synthesis and surface modification of highly monodispersed. spherical gold nanoparticles of 50–200 nm. J. Am. Chem. Soc. 2009;131:17042–17043. doi: 10.1021/ja907069u. [DOI] [PubMed] [Google Scholar]
- [51].Zhang L D, Du X W, Su Y, Niu S Q, Li Y Q, Liang X H, Luo H M. Quantitative assessment of AD markers using naked eyes: Point-of-care testing with paper-based lateral flow immunoassay. J. Nanobiotechnol. 2021;19:366. doi: 10.1186/s12951-021-01111-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhang L D, Yang C W, Li Y Q, Niu S Q, Liang X H, Zhang Z H, Luo Q M, Luo H M. Dynamic changes in the levels of amyloid-β42 species in the brain and periphery of APP/PS1 mice and their significance for Alzheimer’s disease. Front. Mol. Neurosci. 2021;14:723317. doi: 10.3389/fnmol.2021.723317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Qu W, Zhang L, Liang X, Yu Z, Huang H, Zhao J, Guo Y, Zhou X, Xu S, Luo H, et al. Elevated plasma oligomeric amyloid β-42 is associated with cognitive impairments in cerebral small vessel disease. Biosensors (Basel). 2023;13:110. doi: 10.3390/bios13010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Abramoff M D, Magelhaes P J, Ram S J. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- [55].Zhang L D, Du X W, Chen Z X, Chen C J, Gong N X, Song Y H, Song Y Z, Han Q Q, Xia X S, Luo H M, et al. Instrument-free and visual detection of salmonella based on magnetic nanoparticles and an antibody probe immunosensor. Int. J. Mol. Sci. 2019;20:4645. doi: 10.3390/ijms20184645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hu Y Y, He S S, Wang X C, Duan Q H, Grundke-Iqbal I, Iqbal K, Wang J Z. Levels of nonphosphorylated and phosphorylated tau in cerebrospinal fluid of Alzheimer’s disease patients: An ultrasensitive bienzyme-substrate-recycle enzyme-linked immunosorbent assay. Am. J. Pathol. 2002;160:1269–1278. doi: 10.1016/S0002-9440(10)62554-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Phan L M T, Cho S. Fluorescent aptasensor and colorimetric aptablot for p-tau231 detection: Toward early diagnosis of Alzheimer’s disease. Biomedicines. 2022;10:93. doi: 10.3390/biomedicines10010093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Luk C, Giovannoni G, Williams D R, Lees A J, de Silva R. Development of a sensitive ELISA for quantification of three- and four-repeat tau isoforms in tauopathies. J. Neurosci. Methods. 2009;180:34–42. doi: 10.1016/j.jneumeth.2009.02.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ultrasensitive and point-of-care detection of plasma phosphorylated tau in Alzheimer’s disease using colorimetric and surface-enhanced Raman scattering dual-readout lateral flow assay