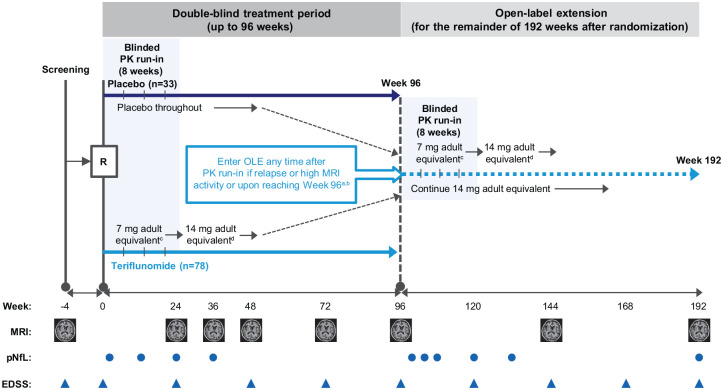
Figure 1.
TERIKIDS study design.
aEntry to OLE any time after initial PK run-in; a new run-in period (8 weeks) starts after entry to extension.
bCriteria for high MRI activity to qualify for switch to the OLE treatment were ⩾9 new/enlarged T2 lesions at Week 36 or ⩾5 new/enlarged T2 lesions on each of two consecutive MRI scans at Weeks 36 and 48, or at Weeks 48 and 72.
cDetermined by body weight category: patients who weighed 20–40 kg received 3.5 mg/day; patients >40 kg received 7 mg/day.
dDetermined by a combination of body weight category and individually predicted PK parameters based on data collected during the run-in period: patients who weighed 20–40 kg received 7 mg/day if predicted PK parameters were equal to or less than the adult range of predicted parameters for a repeated dose of 7 mg, or received 3.5 mg/day if PK parameters were higher than the adult range; patients > 40 kg received 14 mg/day if PK parameters were equal to or less than the adult range of predicted parameters, or received 7 mg/day if PK parameters were higher than the adult range.
EDSS = Expanded Disability Status Scale; MRI = magnetic resonance imaging; OLE = open-label extension; PK = pharmacokinetic; pNfL = plasma neurofilament light chain; R = randomization.