ABSTRACT
Background
Mineralocorticoid receptor antagonists (MRAs) reduce systolic blood pressure (SBP) and increase serum potassium concentration ([K+]). This indirect comparison investigated any differences in SBP-lowering and hyperkalemia risk between finerenone, a nonsteroidal MRA, and the steroidal MRA spironolactone ± a potassium binder.
Methods
In FIDELITY (a pooled analysis of FIDELIO-DKD and FIGARO-DKD), a subgroup of patients with treatment-resistant hypertension (TRH) and chronic kidney disease meeting eligibility criteria of the AMBER trial were identified (FIDELITY-TRH). The main outcomes were mean change in SBP, incidence of serum [K+] ≥5.5 mmol/L and hyperkalemia-associated treatment discontinuation. Results at ∼17 weeks were compared with 12 weeks from AMBER.
Results
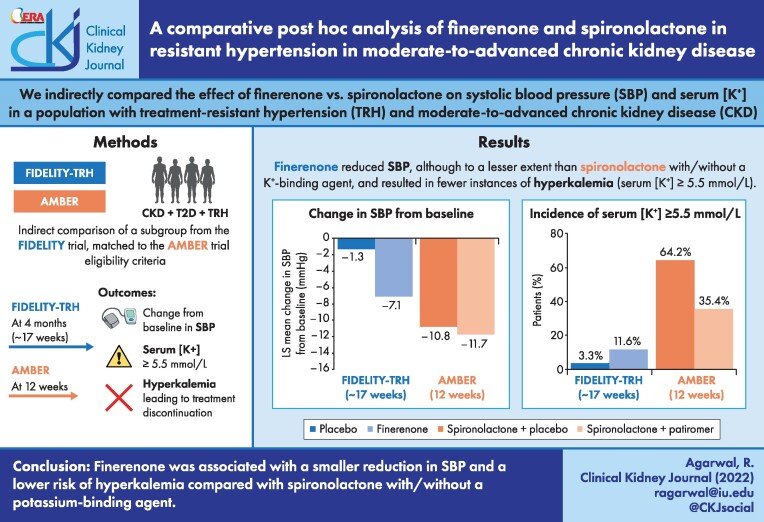
In 624 FIDELITY-TRH patients and 295 AMBER patients, the least squares mean change in SBP (mmHg) from baseline was −7.1 for finerenone and −1.3 for placebo {between-group difference −5.74 [95% confidence interval (CI) −7.99 to −3.49], P < .0001} versus −11.7 for spironolactone + patiromer and −10.8 for spironolactone + placebo [between-group difference −1.0 (95% CI −4.4–2.4), P = .58]. The incidence of serum [K+] ≥5.5 mmol/L was 12% for finerenone and 3% for placebo versus 35% with spironolactone + patiromer and 64% with spironolactone + placebo. Treatment discontinuation due to hyperkalemia was 0.3% for finerenone and 0% for placebo versus 7% for spironolactone + patiromer and 23% for spironolactone + placebo.
Conclusions
In patients with TRH and chronic kidney disease compared with spironolactone with or without patiromer, finerenone was associated with a lower SBP reduction and lower risk of hyperkalemia and treatment discontinuation.
Trial Registration: AMBER (NCT03071263), FIDELIO-DKD (NCT02540993), FIGARO-DKD (NCT02545049)
Keywords: finerenone, hyperkalemia, hypertension, patiromer, spironolactone
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Patients with chronic kidney disease (CKD) and type 2 diabetes (T2D) have a high prevalence of hypertension, with poorly controlled blood pressure (BP) contributing to CKD progression [1, 2]. Short-term studies have demonstrated that steroidal mineralocorticoid receptor antagonists (MRAs) are effective for managing treatment-resistant hypertension (TRH) [3, 4]. However, the use of these agents may be limited in CKD, especially in patients receiving background therapy with renin–angiotensin system (RAS) inhibitors [5]. Current prescribing information for steroidal MRAs limits their use in CKD due to high rates of MRA drug discontinuation observed in trials [6]. Consequently, European and international guidelines only recommend the steroidal MRA spironolactone for treating TRH in patients with an estimated glomerular filtration rate (eGFR) ≥45 mL/min/1.73 m2 and a serum potassium concentration ([K+]) ≤4.5 mmol/L [7, 8].
The AMBER (Spironolactone With Patiromer in the Treatment of Resistant Hypertension in Chronic Kidney Disease) trial was a phase 2, multicenter, randomized, double-blind, placebo-controlled trial in patients with TRH and advanced CKD that demonstrated the use of patiromer, a potassium binder, was associated with less hyperkalemia and fewer drug discontinuations versus placebo [9]. Thus, adding patiromer enabled more patients with TRH and kidney disease to continue spironolactone treatment.
Finerenone, a nonsteroidal MRA, demonstrated kidney protection in patients with advanced CKD in the FIDELIO-DKD (Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease) trial but has not been evaluated in patients with TRH. Concerns have been raised on the potential benefit of finerenone in treating resistant hypertension because of a ‘modest effect on BP and the risk of hyperkalemic events’ [5].
This post hoc analysis evaluated finerenone in patients from FIDELITY (Finerenone in Chronic Kidney Disease and Type 2 Diabetes: Combined FIDELIO-DKD and FIGARO-DKD Trial) with TRH, defined by AMBER eligibility criteria, to determine its efficacy and safety in TRH complicated by advanced CKD in T2D.
MATERIALS AND METHODS
Study design and participants
The designs of the prespecified pooled analysis FIDELITY (N = 13 026) and the individual phase 3, randomized, double-blind, placebo-controlled FIDELIO-DKD and FIGARO-DKD (Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease) studies were published previously [10–12]. Briefly, adults (≥18 years of age) with CKD and T2D with moderate–severe albuminuria receiving optimized RAS inhibitor therapy and serum [K+] ≤4.8 mmol/L at both run-in and screening were eligible [13, 14]. The design of the phase 2, multicenter, randomized, double-blind, placebo-controlled, parallel-group study AMBER has been published previously [15]. The study follow-up schedules, dosing regimens and potassium-monitoring protocols of these two studies are presented in Table S1.
A patient subgroup from FIDELITY (FIDELITY-TRH) meeting the AMBER eligibility criteria was selected for this analysis. FIDELITY-TRH included patients with an eGFR of 25–45 mL/min/1.73 m2 at screening, baseline serum [K+] 4.3–5.1 mmol/L (screening [K+] ≤4.8 mmol/L, per the FIDELIO-DKD and FIGARO-DKD inclusion criteria) and TRH [mean unattended automated office systolic BP (SBP) 135–160 mmHg at baseline] receiving three or more antihypertensives, including a diuretic, and without nonsteroidal anti-inflammatory drugs or serum [K+]–affecting medications at baseline.
Outcomes
In a matched patient population, FIDELITY-TRH safety and efficacy outcomes at 120 days (∼17 weeks) were compared with 12-week results from AMBER, as this was the closest available dataset. Outcomes included changes from baseline to month 4 in office SBP and urinary albumin:creatinine ratio (UACR), hyperkalemia incidences (defined as serum [K+] ≥5.5 mmol/L), hyperkalemia leading to treatment discontinuation, treatment discontinuation for any reason and other safety events [e.g. hypotension and worsening renal function adverse events (AEs)].
Statistical analyses
Time-to-event analyses were conducted using the safety analysis set (patients who received at least one dose of the study drug), unless stated otherwise. Cumulative incidences for serum [K+] ≥5.5 mmol/L utilized Kaplan–Meier estimates. Changes in mean BP were evaluated using a mixed-model analysis with the factors treatment group, study, baseline value, time, treatment*time, baseline value*time and treatment*study interaction as covariates. Changes in mean UACR from baseline to month 4 were analyzed using the full analysis set.
RESULTS
Patients
A total of 624 FIDELITY patients presented with TRH and advanced CKD. While baseline age, serum [K+], eGFR, SBP and antihypertensive medications were generally similar between the FIDELITY-TRH and comparator population from AMBER, there were some notable differences (Table 1). These included higher median UACR in FIDELITY-TRH versus AMBER patients {647 mg/g [interquartile range (IQR) 227–1424] for finerenone and 605 mg/g [IQR 186–1409] for placebo; and 87 mg/g [IQR 18–467] for spironolactone + patiromer and 73 mg/g [IQR 19–400] for spironolactone + placebo; Table 1}. Furthermore, there was a greater proportion of patients with diabetes in the FIDELITY-TRH population than the comparator (100% versus ∼50%) and a lower proportion with heart failure (HF; 11% versus ∼45%). The comparator study recruited largely from sites in Europe and the USA, whereas FIDELITY recruited from 48 countries worldwide, thus FIDELITY-TRH included a larger Black population (5% versus 1%, respectively) and fewer women than the comparator population (35% versus 48%, respectively). The mean daily dose in the FIDELITY-TRH subgroup was 12.0 mg for finerenone versus 12.5 mg for placebo and the mean cumulative dose administered was 1444 mg versus 1505 mg, respectively, over the course of ∼17 weeks. At ∼17 weeks, 43% and 50% of patients on finerenone and placebo, respectively, received the higher study dose regimen (20 mg once daily [od]). In AMBER, the cumulative spironolactone dose was 2942 versus 2581 mg in the patiromer and placebo groups, respectively. At 12 weeks, 69% and 51% of patients in the patiromer and placebo groups, respectively, were treated with the higher dose of spironolactone (50 mg od).
Table 1:
Baseline characteristics for FIDELITY-TRH and external comparator (AMBER) populations.
| FIDELITY-TRH subgroup | AMBER [9] | |||
|---|---|---|---|---|
| Characteristics | Finerenone (n = 316) | Placebo (n = 308) | Spironolactone + patiromer (n = 147) | Spironolactone + placebo (n = 148) |
| Age (years), mean (SD) | 68 (8) | 68 (9) | 68 (12) | 69 (11) |
| Female, n (%) | 110 (35) | 109 (35) | 71 (48) | 71 (48) |
| Race, n (%) | ||||
| White | 240 (76) | 236 (77) | 145 (99) | 145 (98) |
| Black | 17 (5) | 17 (6) | 2 (1) | 2 (1) |
| Othera | 59 (19) | 55 (18) | 0 | 1 (1) |
| Weight (kg), mean (SD) | 91 (19) | 90 (17) | 82.6 (15.5) | 83.5 (14.4) |
| SBP (mmHg), mean (SD) | 146 (7) | 146 (7) | 143 (7)b | 145 (7.0)b |
| Serum [K+] (mmol/L), n (%) | 4.6 (0.2) | 4.6 (0.2) | 4.7 (0.4) | 4.7 (0.4) |
| <4.3 | – | – | 7 (5) | 17 (11) |
| 4.3–<4.7 | 205 (65) | 190 (62) | 55 (37) | 52 (35) |
| 4.7–5.1 | 111 (35) | 118 (38) | 65 (44) | 65 (44) |
| >5.1 | – | – | 20 (14) | 14 (9) |
| eGFR (mL/min/1.73 m2), mean (SD) | 37 (8) | 36 (7) | 35 (7) | 36 (8) |
| UACR (mg/g), median (IQR) | 647 (227–1424) | 605 (186–1409) | 87 (18–467)c | 73 (19–400)c |
| Creatinine (µmol/L), median (IQR) | 157 (32) | 160 (33) | 150 (129–177) | 152 (129–173) |
| Serum creatinine (µmol/L), median (IQR) | – | – | 150.3 (129.1–176.8) | 151.6 (129.1–173.3) |
| Diabetes, n (%) | 316 (100) | 308 (100) | 73 (50) | 72 (49) |
| Medical history, n (%) | ||||
| Cardiovascular disease | 158 (50) | 171 (56) | – | – |
| Stroke or cerebrovascular accident | – | – | 14 (10) | 15 (10) |
| Ischemic stroke | 33 (10) | 45 (15) | – | – |
| Myocardial infarction | 55 (17) | 61 (20) | 31 (21) | 26 (18) |
| Heart failure | 36 (11) | 35 (11) | 63 (43) | 69 (47) |
| Atrial fibrillation | – | – | 11 (7) | 17 (11) |
| Atrial fibrillation and atrial flutter | 29 (9) | 22 (7) | – | – |
| Number of antihypertensive medications, mean (SD) | 4 (1) | 4 (1) | 4 (0.9) | 4 (0.7) |
| Antihypertensive medications, n (%) | – | – | 4 (3–4) | 3 (3–4) |
| Beta-blocker | 205 (65) | 214 (70) | 87 (59) | 86 (58) |
| Calcium channel blocker | 229 (73) | 211 (69) | 107 (73) | 106 (72) |
| Diuretic | 316 (100) | 308 (100) | 146 (99) | 145 (98) |
| RAS inhibitor | 316 (100) | 308 (100) | 147 (100) | 147 (99) |
| Antidiabetic medication, n (%) | 198 (63) | 208 (68) | 69 (47) | 68 (46) |
FIDELITY-TRH population refers to patients with TRH and moderate–advanced CKD; safety analysis set.
SD, standard deviation.
‘Other’ includes Asian, American Indian, Alaskan native, native Hawaiian or other Pacific islander, not reported or multiple (patients who reported belonging to more than one race).
Systolic automated office BP.
24-h UACR.
Office SBP
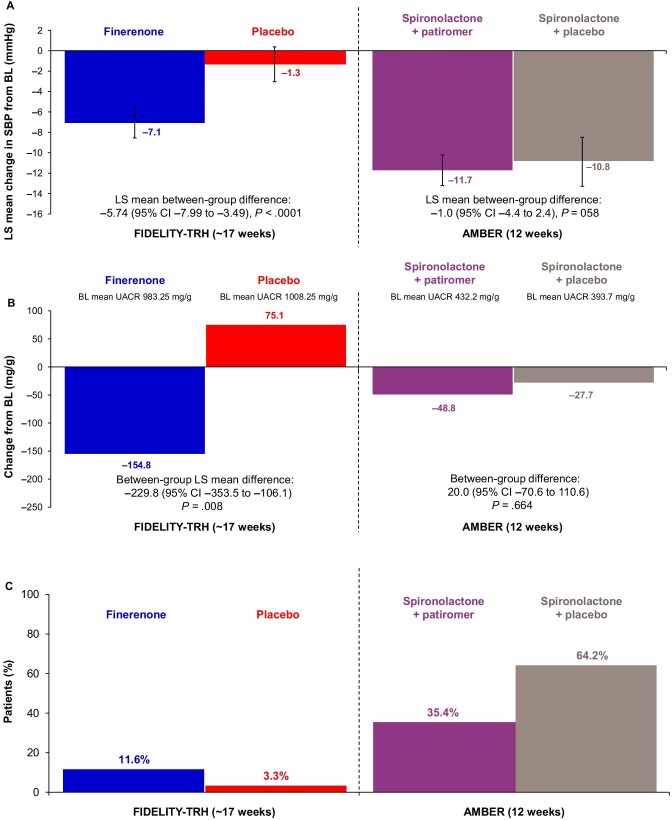
In FIDELITY-TRH, the least squares (LS) mean change from baseline to week 17 in office SBP was −7.1 mmHg [95% confidence interval (CI) −8.6 to −5.6] with finerenone and −1.3 mmHg (95% CI −3.0 to −0.4) with placebo [between-group difference −5.74 (95% CI −7.99 to −3.49), P < .0001; Fig. 1A]. In the comparator study, LS mean changes from baseline to week 12 in automated office SBP were −11.7 mmHg (95% CI −14.1 to −9.3) with spironolactone + patiromer and −10.8 mmHg (95% CI −13.2 to −8.3) with spironolactone and placebo [between-group difference −1.0 (95% CI −4.4–2.4), P = .58; Fig. 1A].
Figure 1:
Outcomes for finerenone versus placebo in FIDELITY-TRH and for spironolactone ± patiromer in AMBER. (A) Office SBP, (B) UACR,a (C) and hyperkalemia outcomes for finerenone versus placebo in the FIDELITY-TRH subgroup (TRH with moderate–advanced CKD population) at ∼17 weeks (120 days) and for spironolactone ± patiromer in AMBER at 12 weeks (serum [K+] >5.5 mmol/L). BL, baseline. aIn AMBER, the between-group difference for UACR corresponds to the difference between groups [(spironolactone + patiromer) – (spironolactone + placebo)].
UACR
The mean UACR at baseline was 983 ± 989 mg/g and 809 ± 1007 mg/g at month 4 (−155 mg/g mean change from baseline to month 4) with finerenone and 1008 ± 1129 mg/g and 1087 ± 1312 mg/g at baseline and month 4 (+75 mg/g mean change from baseline to month 4) with placebo in FIDELITY-TRH patients. The LS mean between-group difference was −229.8 (95% CI −353.5 to −106.1, P = .0082; Fig. 1B). In the comparator study, the mean UACR at baseline and week 12 was 394 and 337 mg/g for patients receiving spironolactone and placebo (−49 mg/g change from baseline to week 12) and 432 and 399 mg/g for patients receiving spironolactone + patiromer (−28 mg/g change from baseline to week 12), respectively. The difference between groups was 20.0 mg/g (95% CI −70.6–110.6).
Hyperkalemia (central laboratory serum [K+] ≥5.5 mmol/L)
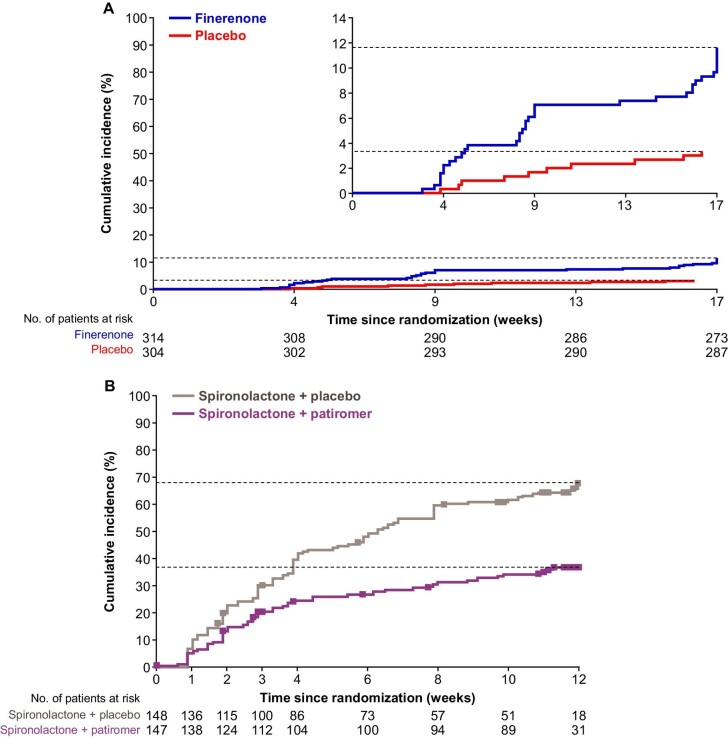
In FIDELITY-TRH, the cumulative incidence of serum [K+] ≥5.5 mmol/L was 12% for finerenone and 3% for placebo after ∼17 weeks (Fig. 1C). In the comparator study, the incidence of serum [K+] ≥5.5 mmol/L was 35% with spironolactone + patiromer and 64% with spironolactone + placebo (P < .001) after 12 weeks [16]. In FIDELITY-TRH, it took fewer days for patients receiving finerenone to reach a serum [K+] ≥5.5 mmol/L than patients receiving placebo (Fig. 2A). In the comparator study, time to serum [K+] ≥5.5 mmol/L was prolonged with spironolactone + patiromer compared with spironolactone + placebo (Fig. 2B). Confounding effects that may have influenced the observed results are reported in Table S2.
Figure 2:
Time to serum [K+] ≥5.5 mmol/L in the FIDELITY-TRH and AMBER populations. Time to serum [K+] ≥5.5 mmol/L in the (A) FIDELITY-TRH (patients with TRH and moderate–advanced CKD; safety analysis set) and (B) external comparator (AMBER) populations. FIDELITY data (A) are cumulative incidences based on Kaplan–Meier estimates. All interruptions were excluded from the person–time at risk and calculation of relative days, i.e. for patients with an interruption, events in the period from interruption start + 3 days until the end of interruption are not considered. For the external comparator AMBER (B), squares indicate censored observations. Patients who did not have any event were censored on the last date with a serum [K+] assessment. Agarwal R, et al. Lancet 2019;394:1540.
Treatment discontinuation
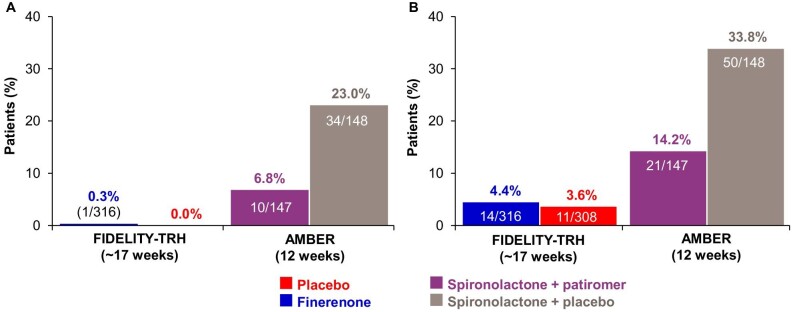
Treatment discontinuation due to hyperkalemia was lower in FIDELITY-TRH versus the comparator [0.3% (1/316) for finerenone and 0% for placebo versus 7% (10/147) for spironolactone + patiromer and 23% (34/148) for spironolactone + placebo; Fig. 3A]. Treatment discontinuation for any reason was lower in FIDELITY-TRH versus the comparator [4% (14/316) for finerenone and 4% (11/308) for placebo over a period of ∼17 weeks versus 14% (21/147) for spironolactone + patiromer and 34% (50/148) for spironolactone + placebo over a period of 12 weeks; Fig. 3B].
Figure 3:
Treatment discontinuation in the FIDELITY-TRH and AMBER populations. (A) Discontinuation due to hyperkalemia and (B) discontinuation for any reason for finerenone versus placebo in the FIDELITY-TRH population (patients with TRH and moderate–advanced CKD) at ∼17 weeks (120 days) versus the external comparator study (AMBER) at 12 weeks.
Other safety events
Serious AEs were reported more frequently in FIDELITY-TRH in both the finerenone (6%) and placebo (6%) groups compared with spironolactone + patiromer (1%) and spironolactone + placebo (3%). Discontinuations due to AEs were lower in FIDELITY-TRH patients in the finerenone (2.2%) and placebo (1%) groups versus spironolactone + patiromer (7%) and spironolactone + placebo (14%; Table 2). Hypotension was also less frequent for finerenone in FIDELITY-TRH patients versus patients in AMBER (2% and 1% for finerenone and placebo, respectively, versus 6% and 4% for spironolactone + patiromer and spironolactone + placebo, respectively; Table 2). Investigator-reported worsening kidney function was less frequent with finerenone in FIDELITY-TRH than with spironolactone ± patiromer in the comparator study (6% and 2% for finerenone and placebo, respectively, versus 12% and 9% for spironolactone + patiromer and spironolactone + placebo, respectively; Table 2). In FIDELITY-TRH, 7% (22/314) and 7% (20/304) of patients receiving finerenone and placebo, respectively, had an eGFR decrease ≥30%, while 19% (28/147) of patients on spironolactone + patiromer and 18% (26/148) of patients on spironolactone + placebo experienced an eGFR decrease≥30% in AMBER (Table 2).
Table 2:
Treatment-emergent AEs from baseline to month 4 for FIDELITY-TRH and the external comparator (AMBER) populations.
| FIDELITY-TRH subgroup | AMBER [9] | |||
|---|---|---|---|---|
| AEs | Finerenone (n = 316) | Placebo (n = 308) | Spironolactone + patiromer (n = 147) | Spironolactone + placebo (n = 148) |
| Any AE | 144 (45.6) | 162 (52.6) | 82 (55.8) | 79 (53.4) |
| Severe | 12 (3.8) | 14 (4.5) | 2 (1.4) | 3 (2.0) |
| Leading to discontinuation | 7 (2.2) | 3 (1.0) | 10 (6.8) | 21 (14.2) |
| Any SAE | 19 (6.0) | 17 (5.5) | 1 (0.7) | 4 (2.7) |
| AE with outcome of death | 1 (0.3) | 0 | 0 | 1 (0.7) |
| Hypotension | 5 (1.6) | 3 (1.0) | 9 (6.1) | 6 (4.1) |
| Leading to discontinuation | 0 (0) | 0 (0) | 4 (2.7) | 2 (1.4) |
| Worsening renal function | 19 (6.0) | 6 (1.9) | 17 (11.6) | 14 (9.5) |
| Leading to discontinuation | 3 (0.9) | 0 (0.0) | 2 (1.4) | 3 (2.0) |
| eGFR decrease ≥30% | 22/314 (7.0) | 20/304 (6.6) | 28 (19.0) | 26 (17.6) |
| eGFR decrease ≥50% | 3/314 (1.0) | 2/304 (0.7) | 1 (0.70) | 4 (2.7) |
FIDELITY-TRH population refers to patients with TRH and moderate–advanced CKD; safety analysis set. Thirty days were considered as 1 month. All interruptions were excluded from the person–time at risk and calculation of relative days, i.e. for patients with an interruption, events in the period from interruption start + 3 days until end of interruption were not considered.
SAE, serious AE.
DISCUSSION
TRH is common in patients with CKD and has been associated with an increased risk of adverse cardiorenal outcomes [17]. In this subgroup of FIDELITY patients with TRH and moderate–advanced CKD, those receiving finerenone had less SBP reduction, greater UACR reduction, lower incidence of hyperkalemia and lower risk of worsening kidney function compared with patients receiving spironolactone with patiromer or placebo in the external comparator study.
FIDELITY-TRH patients had a smaller reduction (baseline to month 4) in SBP than spironolactone ± patiromer patients, likely because of higher achievement of the target dose in AMBER, different modes of action or the very long half-life and active metabolites of spironolactone [18, 19]. Additionally, all patients in the comparator study received open-label spironolactone without an untreated control group, whereas FIDELITY was placebo controlled. Of note, the placebo-corrected SBP-lowering effects of finerenone in FIDELITY-TRH patients were larger than the modest effect reported in the overall FIDELITY population (−5.7 versus −3.2 mmHg at weeks 17 and 16, respectively), likely because of the higher baseline BP in the TRH subgroup investigated [10].
The absolute UACR reduction with finerenone in FIDELITY-TRH exceeded that of spironolactone in the comparator study. This may reflect the lower overall mean UACR in the comparator versus FIDELITY-TRH population (Table 1; Table S2). Prior meta-analyses show that spironolactone is effective in lowering UACR in patients with elevated urine albumin excretion rates [20]. Further data are needed to compare the effect of the two treatments on UACR.
Steroidal MRAs are used infrequently in patients with CKD, and their initiation rates remain low in patients with HF and CKD (26%, 35% and 40% in patients with eGFRs of 30–45, 45–60 and 60–<90 mL/min/1.73 m2, respectively), likely because of the increased hyperkalemia risk and high rates of drug discontinuation [6, 7, 21]. Therefore the nonsteroidal MRA finerenone can overcome these limitations even in this very high–risk group of patients with advanced CKD and T2D with moderately or severely elevated albuminuria and is approved for initiation down to an eGFR of 25 mL/min/1.73 m2 in patients with T2D and CKD.
The ∼30-day phase 2 ARTS (Mineralocorticoid Receptor Antagonist Tolerability Study) is the only clinical head-to-head comparison of finerenone (2.5–10 mg od and 5 mg twice daily) with spironolactone (25–50 mg od) [22]. In ARTS, finerenone (10 mg od) had a smaller effect than spironolactone (25–50 mg od) on SBP (−4.2 versus −10.1 mmHg change between baseline and day 29; P < .05) [22]. Furthermore, the incidence of investigator-reported hyperkalemia was lower with finerenone than spironolactone (5.3% versus 12.7%, respectively; P = .048). The same was observed for discontinuation due to hyperkalemia [0 versus 2 (3.2%), respectively], although the sample size was small (67 and 63 patients for finerenone 10 mg and spironolactone, respectively) and treatment duration was limited to 1 month.
Together, this FIDELITY analysis and ARTS data suggest that finerenone has a lower risk of hyperkalemia and lower discontinuation rates than spironolactone [22, 23]. This may reflect the distinct mechanism of action for finerenone compared with spironolactone, along with potential differences in tissue distribution that may affect [K+] homeostasis [18, 19, 22, 24]. Differences in plasma half-life (2.2–2.8 h with finerenone compared with >12 h in healthy volunteers with spironolactone, respectively) may also be relevant [18, 19, 22, 24]. Additionally, finerenone has no active metabolites, whereas spironolactone has multiple; in AMBER, 75% and 36% of patients who stopped spironolactone still had metabolites detectable after 2 and 3 weeks, respectively [9, 18]. Finally, the dosing of finerenone and spironolactone differed in the two studies (Table S1).
When considering treatment-emergent AEs from baseline to month 4, the incidence of an eGFR decrease ≥30% was more than double in the comparator versus FIDELITY-TRH group, suggesting that finerenone may have a less acute effect on renal hemodynamics. This is notable because all patients received maximum tolerated doses of RAS inhibitors in FIDELITY, as RAS inhibitors alone have been shown to increase the risk of acute kidney injury [25]. Incidences of worsening renal function and hypotension AEs were lower with finerenone in FIDELITY-TRH patients than with spironolactone with or without patiromer in the comparator study.
Limitations
As mentioned, there was a lower incidence of discontinuation and serum [K+] ≥5.5 mmol/L in FIDELITY-TRH compared with AMBER, likely because serum [K+] monitoring protocols differed. In FIDELIO-DKD and FIGARO-DKD, follow-up visits were conducted at months 1 and 4, then every 4 months [13, 14]. In the comparator study, follow-up visits were scheduled weekly during weeks 1–4 then biweekly during weeks 6–12 [9]. The more frequent follow-up schedule in AMBER may have facilitated increased detection of hyperkalemia, thereby increasing the incidence of discontinuation due to hyperkalemia. However, even with a more frequent follow-up protocol in ARTS [screening visit, baseline/day 1, day 4 ± 1, day 8 ± 1, then weekly until the end of the study, and at follow-up (14 days after the last intake of the study drug)] [22], hyperkalemia was >2 times more common with spironolactone than with finerenone (12.7% and 5.3%, respectively; P = .048) in just 30 days [22].
Other differences may also have influenced the results observed (Table S2). These included differences in serum [K+] at baseline (0.1 mEq/L higher serum [K+] in AMBER) and differences in hyperkalemia risk factors at baseline, including race, sex, diabetes, HF, medication use and baseline UACR because of differences in study design. Patients who are Black are considered less likely to develop hyperkalemia than patients who are White [26, 27]. Similarly, female sex is associated with a lower risk of hyperkalemia [24, 27], whereas increased UACR levels are associated with an increased risk of hyperkalemia [24]. In addition, all FIDELITY-TRH patients had diabetes and more patients were receiving beta-blockers, which have been associated with higher hyperkalemia rates, in contrast to the comparator study [27, 28].
The comparator study was designed to maximize detection of benefits with the potassium-binding agent patiromer to increase spironolactone persistence [9]. In contrast, the FIDELIO-DKD and FIGARO-DKD trials, and therefore FIDELITY-TRH analysis, were designed to maximize detection of finerenone benefits on hard cardiorenal outcomes [10, 13, 14]. Another potentially confounding factor was that the comparator study was open label while FIDELITY was double blind [9]. In the comparator study, it was therefore known that all patients were receiving spironolactone, with stopping rules clearly defined [9]. Consequently, investigators in the comparator study may be more likely to discontinue because of biases based on risks of hyperkalemia associated with known spironolactone use. An additional limitation was that the comparator study was shorter, limiting the length of the comparison.
CONCLUSIONS
Indirect comparison of data from a patient subgroup with TRH and moderate–advanced CKD from FIDELITY with patients from an external comparator study (AMBER) suggests that finerenone may be associated with a lower magnitude of SBP reduction and a lower risk of hyperkalemia during the first 4 months of treatment than spironolactone, with or without a potassium-binding agent.
Supplementary Material
ACKNOWLEDGEMENTS
The authors and study sponsor are indebted to the patients and their families, as well as the investigators and sites participating in the studies. Medical writing assistance was provided by Cindy-Jade Jenner, of Chameleon Communications International, funded by Bayer AG.
Contributor Information
Rajiv Agarwal, Richard L. Roudebush VA Medical Center and Indiana University, Indianapolis, IN, USA.
Bertram Pitt, Department of Medicine, University of Michigan School of Medicine, Ann Arbor, MI, USA.
Biff F Palmer, Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Csaba P Kovesdy, Division of Nephrology, Department of Medicine, University of Tennessee, Health Science Center Memphis, TN, USA; Nephrology Section, Memphis VA Medical Center, Memphis, TN, USA.
Ellen Burgess, Department of Medicine, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada.
Gerasimos Filippatos, National and Kapodistrian University of Athens, School of Medicine, Department of Cardiology, Attikon University Hospital, Athens, Greece.
Jolanta Małyszko, Department of Nephrology, Dialysis and Internal Medicine, Medical University of Warsaw, Warsaw, Poland.
Luis M Ruilope, Cardiorenal Translational Laboratory and Hypertension Unit, Institute of Research imas12, Madrid, Spain; CIBER-CV, Hospital Universitario 12 de Octubre, Madrid, Spain; Faculty of Sport Sciences, European University of Madrid, Madrid, Spain.
Patrick Rossignol, Université de Lorraine, Inserm, Centre d’Investigations Cliniques – Plurithématique 14-33, and Inserm U1116, CHRU Nancy, F-CRIN INI-CRCT (Cardiovascular and Renal Clinical Trialists), Nancy, France.
Peter Rossing, Steno Diabetes Center Copenhagen, Herlev, Denmark; Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
Roberto Pecoits-Filho, Arbor Research Collaborative for Health, Ann Arbor, MI, USA; School of Medicine, Pontificia Universidade Católica do Paraná, Curitiba, Brazil.
Stefan D Anker, Department of Cardiology and Berlin Institute of Health Center for Regenerative Therapies, German Centre for Cardiovascular Research Partner Site Berlin, Charité Universitätsmedizin, Berlin, Germany.
Amer Joseph, Cardiology and Nephrology Clinical Development, Bayer AG, Berlin, Germany.
Robert Lawatscheck, Cardiology and Nephrology Clinical Development, Bayer AG, Berlin, Germany.
Daniel Wilson, US Medical Affairs, Bayer US LLC Pharmaceuticals, Whippany, NJ, USA.
Martin Gebel, Research and Development, Integrated Analysis Statistics, Bayer AG, Wuppertal, Germany.
George L Bakris, Department of Medicine, University of Chicago Medicine, Chicago, IL, USA.
FUNDING
This work was supported by Bayer AG, who funded the FIDELIO-DKD and FIGARO-DKD studies and combined analysis. The funder participated in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication. R.A., M.G. and R.L. had full access to all study data and take responsibility for the integrity of the data and the accuracy of the data analysis.
AUTHORS’ CONTRIBUTIONS
R.A., M.G. and R.L. were responsible for the concept and design. R.A. developed the first draft of the manuscript. M.G. was responsible for the statistical analysis. All authors were responsible for the acquisition, analysis or interpretation of data and critical revision of the manuscript for important intellectual content. R.A., M.G. and R.L. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
DATA AVAILABILITY STATEMENT
Data are not currently available but will be available in an electronic repository. Data availability will begin at a date to be confirmed by Bayer AG.
CONFLICT OF INTEREST STATEMENT
R.A. has received personal fees and nonfinancial support from Bayer Healthcare Pharmaceuticals during the conduct of the study; personal fees and nonfinancial support from Akebia Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Vifor Pharma; is a member of data safety monitoring committees for Chinook and Vertex; served as associate editor of the American Journal of Nephrology and Nephrology Dialysis and Transplantation and as an author for UpToDate; and has received research grants from the US Veterans Administration and the National Institutes of Health.
S.D.A. has received research support from Abbott Vascular and Vifor International and personal fees from Abbott Vascular, Bayer, Boehringer Ingelheim, BRAHMS, Cardiac Dimensions, Impulse Dynamics, Novartis, Servier and Vifor Pharma.
G.F. is a committee member of trials and registries sponsored by Amgen, Bayer, Boehringer Ingelheim, Medtronic, Novartis, Servier and Vifor Pharma and is senior consulting editor for JACC Heart Failure and has received research support from the European Union.
P.R. has received personal fees from Bayer during the conduct of the study, research support and personal fees from AstraZeneca and Novo Nordisk and personal fees from Astellas, Boehringer Ingelheim, Eli Lilly, Gilead, Mundipharma, Sanofi and Vifor; all fees are given to Steno Diabetes Center Copenhagen.
L.M.R. has received consultancy fees from Bayer.
B.P. has received consultancy fees for AstraZeneca, Bayer, Boehringer Ingelheim, Brainstorm Medical, Cereno Scientific, G3 Pharmaceuticals, KBP Biosciences, PhaseBio, Proton Intel, Sanofi/Lexicon, Sarfez, scPharmaceuticals, SQ Innovation, Tricida and Vifor/Relypsa; has stock options for KBP Biosciences, Brainstorm Medical, Cereno Scientific, G3 Pharmaceuticals, Proton Intel, Sarfez, scPharmaceuticals, SQ Innovation, Tricida and Vifor/Relypsa and holds a patent for site-specific delivery of eplerenone to the myocardium (US patent 9931412) and a provisional patent for histone acetylation-modulating agents for the treatment and prevention of organ injury (provisional patent US 63/045784).
C.K. has received consultancy fees from Abbott, Akebia, AstraZeneca, Bayer, Boehringer Ingelheim, Cara Therapeutics, CSL Behring, Rockwell and Vifor and royalties from UpToDate and Springer.
R.P.-F. has received research grants from Fresenius Medical Care and the National Council for Scientific and Technological Development; grants (paid to employer) from Akebia, AstraZeneca, Bayer, Boehringer Lilly and Novo Nordisk for participation in advisory boards and educational activities and is employed by Arbor Research Collaborative for Health, who runs the Dialysis Outcomes and Practice Patterns Study (DOPPS). Global support for the ongoing DOPPS programs is provided without restriction on publications by a variety of funders. Funding is provided to Arbor Research Collaborative for Health and not to R.P.-F. directly. For details, see https://www.dopps.org/AboutUs/Support.aspx.
P.R. has received consultancy fees for Bayer, G3P, Idorsia and KBP; honoraria from Ablative Solutions, AstraZeneca, Bayer, Boehringer Ingelheim, CinCor, Corvidia, CVRx, Fresenius, Grünenthal, Novartis, Novo Nordisk, Roche, Sanofi, Sequana Medical, Servier, Stealth Peptides, Vifor Fresenius Medical Care Renal Pharma and Vifor Pharma, Inc.; personal fees from Ablative Solutions, Bayer, Boehringer Ingelheim, Corvidia, CVRx, Grünenthal, Idorsia, KBP, Novo Nordisk, Sanofi, Sequana Medical, Servier, Stealth Peptides and Vifor Pharma; grants and personal fees from AstraZeneca, Bayer, Fresenius, Novartis and Vifor Fresenius Medical Care Renal Pharma and nonfinancial support from Fresenius; has ownership interest in G3P and is cofounder of CardioRenal and has received research funding from Vifor Fresenius Medical Care Renal Pharma and Vifor Pharma.
B.P. has no financial disclosures.
E.B. is a member of the FIDELIO-DKD steering committee.
J.M. has received grants and/or honoraria for consultancy or lectures from Bayer, AstraZeneca, Astellas and Boehringer Ingelheim.
A.J. was previously a full-time employee of Bayer AG, Pharmaceuticals Division, Germany at the time of the studies and analysis. He is currently a full-time employee of Chiesi Farmaceutici S.p.A, Parma, Italy.
M.G., D.W. and R.L. are full-time employees of Bayer AG, Pharmaceuticals Division, Germany.
G.B. has received research funding, paid to the University of Chicago Medicine, from Bayer, Novo Nordisk and Vascular Dynamics during the conduct of the study; consultancy and personal fees from Alnylam, Merck and Relypsa; is editor of the American Journal of Nephrology, Nephrology and Hypertension, section editor of UpToDate and associate editor of Diabetes Care and Hypertension Research.
REFERENCES
- 1.Ku E, Lee BJ, Wei Jet al. . Hypertension in CKD: core curriculum 2019. Am J Kidney Dis 2019;74:120–31. 10.1053/j.ajkd.2018.12.044. [DOI] [PubMed] [Google Scholar]
- 2.Van Buren PN, Toto R.. Hypertension in diabetic nephropathy: epidemiology, mechanisms, and management. Adv Chronic Kidney Dis 2011;18:28–41. 10.1053/j.ackd.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams B, MacDonald TM, Morant Set al. . Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet 2015;386:2059–68. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(15)00257-3/fulltext. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calhoun DA, White WB.. Effectiveness of the selective aldosterone blocker, eplerenone, in patients with resistant hypertension. J Am Soc Hypertens 2008;2:462–8. 10.1016/j.jash.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes . KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int 2021;99(3 Suppl):S1–87.33637192 [Google Scholar]
- 6.Beldhuis IE, Myhre PL, Claggett Bet al. . Efficacy and safety of spironolactone in patients with HFpEF and chronic kidney disease. JACC Heart Fail 2019;7:25–32. 10.1016/j.jchf.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Williams B, Mancia G, Spiering Wet al. . 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–104. 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 8.Unger T, Borghi C, Charchar Fet al. . 2020 International Society of Hypertension global hypertension practice guidelines. J Hypertens 2020;38:982–1004. 10.1097/hjh.0000000000002453. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal R, Rossignol P, Romero Aet al. . Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2019;394:1540–50. https://www.sciencedirect.com/science/article/pii/S014067361932135X?via%3Dihub. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R, Filippatos G, Pitt Bet al. . Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J 2022;43:474–84. 10.1093/eurheartj/ehab777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakris GL, Agarwal R, Anker SDet al. . Design and baseline characteristics of the Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic kidney Disease trial. Am J Nephrol 2019;50:333–44. 10.1159/000503713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruilope LM, Agarwal R, Anker SDet al. . Design and baseline characteristics of the Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease trial. Am J Nephrol 2019;50:345–56. 10.1159/000503712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakris GL, Agarwal R, Anker SDet al. . Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020;383:2219–29. 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 14.Pitt B, Filippatos G, Agarwal Ret al. . Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021;385:2252–63. 10.1056/NEJMoa2110956. [DOI] [PubMed] [Google Scholar]
- 15.Agarwal R, Rossignol P, Garza Det al. . Patiromer to enable spironolactone use in the treatment of patients with resistant hypertension and chronic kidney disease: rationale and design of the AMBER study. Am J Nephrol 2018;48:172–80. 10.1159/000492622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veltassa (Patiromer) Summary of Product Characteristics. Available from: https://www.ema.europa.eu/documents/product-information/veltassa-epar-product-information_en.pdf [accessed 21 February 2022]. [Google Scholar]
- 17.Fay KS, Cohen DL.. Resistant hypertension in people with CKD: a review. Am J Kidney Dis 2021;77:110–21. 10.1053/j.ajkd.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal R, Kolkhof P, Bakris Get al. . Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J 2021;42:152–61. 10.1093/eurheartj/ehaa736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolkhof P, Jaisser F, Kim SYet al. . Steroidal and novel non-steroidal mineralocorticoid receptor antagonists in heart failure and cardiorenal diseases: comparison at bench and bedside. Handb Exp Pharmacol 2017;243:271–305. https://link.springer.com/chapter/10.1007/164_2016_76. [DOI] [PubMed] [Google Scholar]
- 20.Bolignano D, Palmer SC, Navaneethan SDet al. . Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev 2014;4:CD007004. 10.1002/14651858.CD007004.pub3. [DOI] [PubMed] [Google Scholar]
- 21.Patel RB, Fonarow GC, Greene SJet al. . Kidney function and outcomes in patients hospitalized with heart failure. J Am Coll Cardiol 2021;78:330–43. 10.1016/j.jacc.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pitt B, Kober L, Ponikowski Pet al. . Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J 2013;34:2453–63. 10.1093/eurheartj/eht187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuo C, Xu G.. Efficacy and safety of mineralocorticoid receptor antagonists with ACEI/ARB treatment for diabetic nephropathy: a meta-analysis. Int J Clin Pract 2019;73:e13413. 10.1111/ijcp.13413. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal R, Joseph A, Anker SDet al. . Hyperkalemia risk with finerenone: results from the FIDELIO-DKD trial. J Am Soc Nephrol 2022;33:225–37. 10.1681/ASN.2021070942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fried LF, Emanuele N, Zhang JHet al. . Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med 2013;369:1892–903. 10.1056/NEJMoa1303154. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal R. Modification of potassium-mortality relationship by ethnicity and race: solving the puzzle. Am J Nephrol 2017;45:552–4. 10.1159/000476004. [DOI] [PubMed] [Google Scholar]
- 27.Kovesdy CP, Matsushita K, Sang Yet al. . Serum potassium and adverse outcomes across the range of kidney function: a CKD prognosis consortium meta-analysis. Eur Heart J 2018;39:1535–42. 10.1093/eurheartj/ehy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandić D, Nezić L, Škrbić R. Severe hyperkalemia induced by propranolol. Med Pregl 2014;67:181–4. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are not currently available but will be available in an electronic repository. Data availability will begin at a date to be confirmed by Bayer AG.