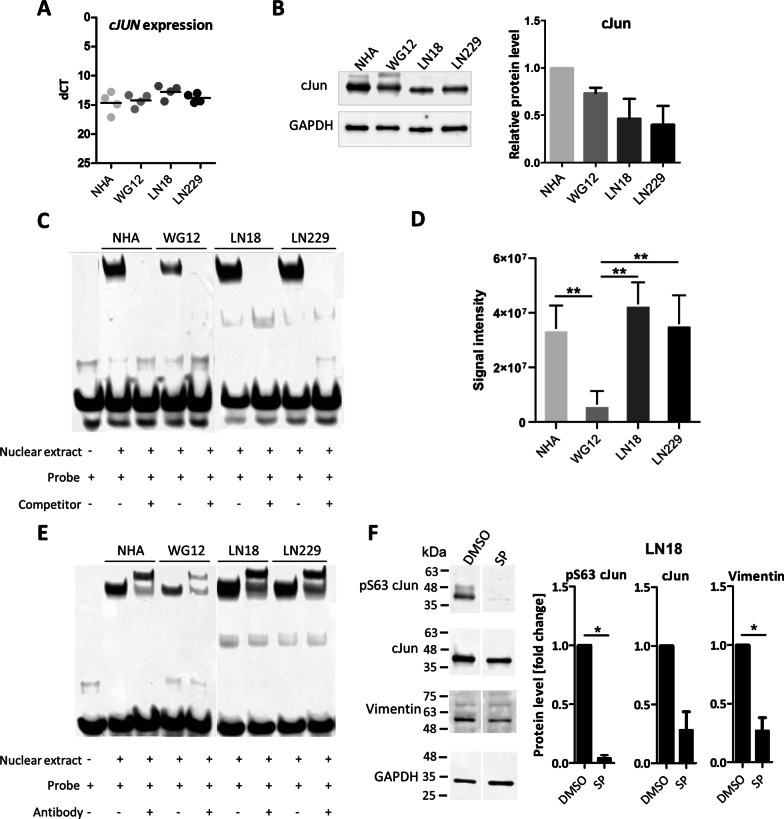
Fig. 5.
c-Jun transcription factor binds to the VIM promoter in human astrocytes and glioma cell lines. c-Jun levels in normal human astrocytes (NHA), low-grade glioma patient-derived cell cultures (WG12) and established glioma cell lines (LN18, LN229). A c-JUN mRNA expression was evaluated by RT-qPCR. Data were normalized to the expression of GAPDH mRNA determined in the same sample, n = 4. B Protein levels of c-Jun analyzed by Western blot with the densitometry of immunoblots. Data were normalized to the levels of GAPDH in the same sample, n = 3, mean± SD. The densitometry is presented as relative values to NHA set as 1. P values were calculated using GraphPad software and considered significant when *p < 0.05 (One-way ANOVA). C DNA-binding activity of double-stranded DNA from the Vimentin promoter site. EMSA was performed using the LightShift Chemiluminescent EMSA Kit. Nuclear extracts were isolated from NHA, WG12, LN18 and LN229. Unlabeled competitor probes were added to lanes 3, 5, 7, and 9, n = 3. D Densitometry analysis of EMSA presented as signal intensity mean ± SD. One-way ANOVA with Dunnett’s post hoc test revealed significant differences at **p < 0.01, n = 3. E Supershift EMSA assay for measuring c-Jun transcription factor binding to DNA from the Vimentin promoter. Antibody against c-Jun was added to samples in lanes 3, 5, 7, and 9 to verify if the observed shift of the probe band in the gel was dependent on c-Jun binding, n = 3. F Impact of inhibition of c-Jun phosphorylation on the level of Vimentin. LN18 cells were treated for 3 h with SP600125 (SP), an inhibitor of JNK kinases. Protein levels were analyzed by Western blot with the densitometry analysis. Data are presented as relative values to control (cells treated with DMSO, set as 1). Data were normalized to the levels of GAPDH in the same sample. P values were calculated using GraphPad software and considered significant when *p < 0.05 (t-test), n = 3, ± SD