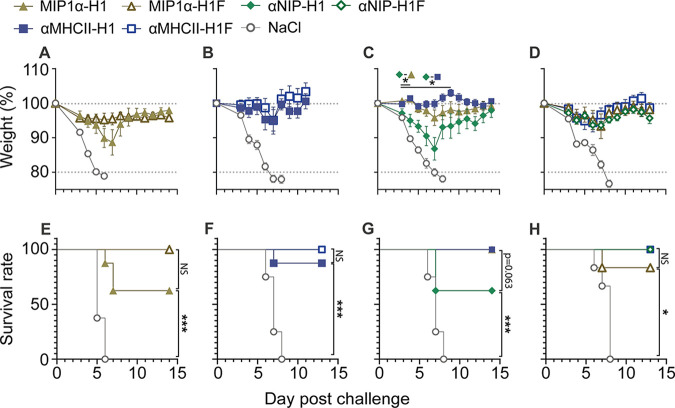
FIG 4.
Vaccine effectiveness against a lethal influenza challenge. Mice were vaccinated with a single dose of the indicated vaccines and challenged 7 to –13 weeks postvaccination with a 5 × LD50 dose of influenza PR8 intranasally (i.n.) (n = 8 [panels A to C and E to G] or 6 [panels D and H] mice/group). Mice were monitored daily for weight loss; data are displayed as the weight mean ± SEM (panels A to D), with a humane endpoint of 20% body weight loss as basis for survival curves (panels E to H). (A and B) Comparison of vaccines with or without a trimerization domain: (A) weight after PR8 challenge 10 weeks postvaccination with MIP1α vaccines and (B) weight after challenge 7 weeks postvaccination with MHCII-targeted vaccines. (C and D) Comparison of different APC-specific targeting moieties: weight following viral challenge is shown for vaccines either (C) without a trimerization domain 10 weeks after vaccination or (D) with a trimerization domain 13 weeks after vaccination. Significant weight loss was determined by group-wise comparison using a two-sided Mann-Whitney test for each time point. Significance between vaccine groups is shown above the corresponding time point; *, P < 0.05; **, P < 0.005. (E to H) Survival curves corresponding to the above-described weight panels. Significance was calculated by log-rank (Mantel-Cox) tests. *, P < 0.0332; **, P < 0.0021; ***, P < 0.0002.