Abstract
Introduction
The data of acute kidney injury (AKI), that is, community-acquired AKI (CA-AKI) and hospital-acquired AKI (HA-AKI) among non-COVID patients from intensive care units (ICU) during the coronavirus disease-2019 (COVID-19) pandemic are scarce. We planned to study the change in the profile of such patients compared to the pre-pandemic era.
Materials and methods
This prospective observational study was conducted at four ICUs dealing with non-COVID patients at a government hospital in North India, and was aimed at assessing outcomes, and mortality predictors of AKI among non-COVID patients during the COVID-19 pandemic. Renal and patient survival at ICU transfer-out and hospital discharge, ICU and hospital stay duration, mortality predictors, and dialysis requirement at discharge were evaluated. The current or previous COVID-19 infection, previous AKI or chronic kidney disease (CKD), organ donors, and organ transplant patients were excluded.
Results
Among the 200 non-COVID-19 AKI patients, diabetes mellitus (DM), primary hypertension, and cardiovascular diseases were the predominant comorbidities in descending order. The commonest cause of AKI was severe sepsis, followed by systemic infections and post-surgery patients. Dialysis requirements at ICU admission during ICU stay and above 30 days were seen in 20.5, 47.5, and 6.5% of patients, respectively. Incidence of CA-AKI and HA-AKI was 1.24:1, whereas dialysis requirement above 30 days was 0.85:1, respectively. The 30-day mortality was 42%. Hepatic dysfunction [hazard ratio (HR): 3.471], septicemia (HR: 3.342), age above 60 years (HR: 4.000), higher sequential organ failure assessment (SOFA) score (HR: 1.107; p = 0.001), anemia (p = 0.003), and low serum iron (p = 0.001) were important mortality predictors in AKI.
Conclusion
Compared to the pre-COVID era, CA-AKI was more common than HA-AKI due to restricted elective surgeries during the COVID-19 pandemic. Acute kidney injury with multiorgan involvement and hepatic dysfunction, elderly age with higher SOFA score and sepsis were predictors of adverse renal and patient outcomes.
How to cite this article
Singh B, Dogra PM, Sood V, Singh V, Katyal A, Dhawan M, et al. Spectrum, Outcomes, and Mortality Predictors of Acute Kidney Injury among Non-COVID-19 Patients during COVID-19 Pandemic: Data from Four Intensive Care Units. Indian J Crit Care Med 2023;27(2):119–126.
Keywords: Acute kidney injury, Dialysis, Non-coronavirus disease-2019, Renal survival
Introduction
Acute kidney injury is a syndrome defined by an abrupt decrease in glomerular filtration with/without a reduction in urine output. About 3–7% of hospitalized and 25–30% of ICU patients develop AKI, with 5–6% among them requiring renal replacement therapy (RRT) after developing AKI.1 In the ICU setting, AKI is associated with high mortality, longer hospital stay, and substantial health resource utilization.2 There is an increased risk for progression to CKD and subsequent advancement of disease to CKD stage V, even after recovery from AKI.3
The etiologic spectrum of HA-AKI in developing countries is like that of developed countries. Acute kidney injury in tropical, low and middle-income countries such as India is characterized by a higher burden of CA-AKI, affecting relatively younger patients without comorbidities and having lower mortality (if diagnosed early) whereas HA-AKI is more common in the developed world, with higher mortality.2,4 In 2013, the International Society of Nephrology launched the “0by25” initiative with the aim to eliminate preventable deaths from AKI worldwide by 2025.5,6 As there is no nationwide AKI registry in India, the data on AKI in critically ill patients is limited to single-center studies.4,7–9
During the present COVID-19 pandemic, specially designated COVID-19 ICUs were established in all countries, and there were numerous publications regarding COVID-19-related AKI. However, the data on AKI among non-COVID-19 patients during the COVID-19 pandemic is scarce. We conducted this prospective study to determine spectrum, outcomes, and mortality predictors of AKI among non-COVID-19 patients in four critical care units during the current pandemic.
Materials and Methods
This prospective observational study enrolled all COVID-19-negative adult patients (aged 18 years or above) getting admitted to four ICUs (two surgical and two medical ICUs) with the diagnosis of AKI. The study period ranged from February 2020 to October 2021. This study was conducted at a tertiary care government hospital in north India. Kidney disease – improving global outcomes (KDIGO) criterion was used for defining AKI.10 Patients with a history of recent/ongoing COVID-19 infection, past COVID-19 infection, CKD, voluntary organ donation, kidney transplantation, previous history of AKI or dialysis were excluded. The study protocol was reviewed and approved by the institutional review committee and informed consent was provided by patients or their legally authorized representatives in case the patient was medically unable to do so. This study was in accordance with the institutional ethical standards, the national research committee, and amendments to the Helsinki Declaration. Clinical trial registration was not done as this was a prospective observational study.
The patients were categorized into CA-AKI and HA-AKI; CA-AKI was defined as AKI diagnosis at admission or within 48 hours of admission, whereas HA-AKI was defined as AKI beyond 48 hours of hospitalization. All patients were followed for 30 days from enrollment. A predesigned standardized proforma was designed to record demographic details including age, gender, comorbid conditions, probable etiology of AKI, hemodynamic parameters, other organs involvement, biochemical parameters at admission and at diagnosis of AKI; urine output; need for mechanical ventilation; need for fluid replacement; need, type, and the number of sessions of RRT; and laboratory parameters. Primary outcome measures for this study were 30-day mortality and above 30 days requirement of RRT. Secondary outcomes were estimated glomerular filtration rate (eGFR) and serum creatinine at transfer-out from ICU and at discharge from the hospital, days of RRT requirement, days of ICU and hospital stay, and serum albumin at discharge, and evaluation of mortality predictors in AKI.
Statistical analyses were performed using the statistical package for social sciences software (SPSS), version 20.0. The probability value was fixed at 0.05 or less. The sample size was calculated with a margin of error (α error) of 5%, a confidence interval (CI) of 95% (Z-score =1.96), the prevalence of AKI in our ICU at any given time (p) as 15% and absolute allowed error (d) of 5%. Using Cochran's formula, 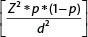 the sample size for adequate power was 196. Hence, a round figure of 200 patients was taken for this study. We stopped enrollment after 200 patients. Descriptive statistics were expressed as percentages and continuous variables as mean with standard deviation or median with range. For outcome, survival status was categorized into survivors and non-survivors groups. The normality of data was assessed using Kolmogorov–Smirnov test. Pearson Chi-squared test or Fisher's exact test, as appropriate was used to assess the association, and the strength of the association was assessed using the spearman correlation test. Mann–Whitney U test was used to compare the survivors and non-survivors with respect to the laboratory parameters. Kaplan–Meier survival curves and log-rank tests were used to compare survival between types of AKI and recovered/died at 30 days with respect to the days in ICU and hospital. Cox regression analysis was performed to calculate HR with respect to factors such as comorbidities, etiology, laboratory parameters, and covariates (age-group, AKI staging) affecting the time to event, that is, death. Multivariate Cox regression analysis was done to find out the most important factor predicting the time to event (death) among the significant factors with p < 0.05 in univariate analysis.
the sample size for adequate power was 196. Hence, a round figure of 200 patients was taken for this study. We stopped enrollment after 200 patients. Descriptive statistics were expressed as percentages and continuous variables as mean with standard deviation or median with range. For outcome, survival status was categorized into survivors and non-survivors groups. The normality of data was assessed using Kolmogorov–Smirnov test. Pearson Chi-squared test or Fisher's exact test, as appropriate was used to assess the association, and the strength of the association was assessed using the spearman correlation test. Mann–Whitney U test was used to compare the survivors and non-survivors with respect to the laboratory parameters. Kaplan–Meier survival curves and log-rank tests were used to compare survival between types of AKI and recovered/died at 30 days with respect to the days in ICU and hospital. Cox regression analysis was performed to calculate HR with respect to factors such as comorbidities, etiology, laboratory parameters, and covariates (age-group, AKI staging) affecting the time to event, that is, death. Multivariate Cox regression analysis was done to find out the most important factor predicting the time to event (death) among the significant factors with p < 0.05 in univariate analysis.
Results
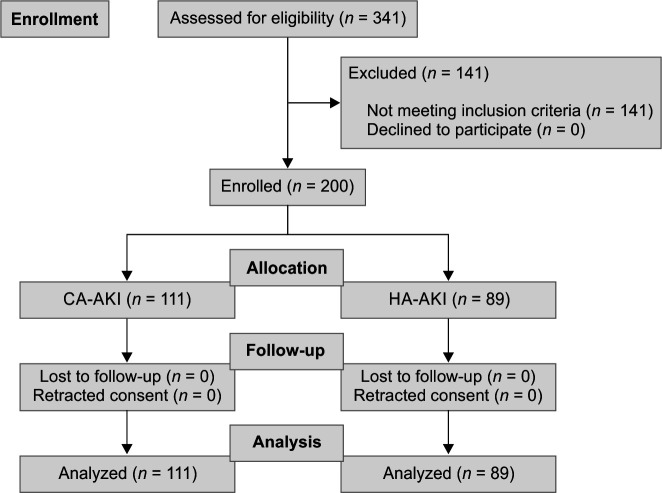
A total of 200 patients were recruited into the study from four ICUs—one medical, one surgical, one cardiology, and one cardiothoracic surgery ICUs (Flowchart 1). The mean age of the cohort was 55.64 ± 17.04 years. About 28.5% were females and 68.5% had AKI-KDIGO stage III. Urine output-based criteria for AKI was used in 6.5% of patients only whereas the rest of the patients had AKI diagnosed by serum creatinine criteria with or without urine output criteria. The etiological risk factors for AKI and co-morbid conditions associated with AKI are shown in Tables 1 and 2. Severe sepsis (33%), infections (32.5%), post-surgery (22.5%), and neurological causes (17%) were the major etiologies, though there was an overlap in several patients. Acute pancreatitis (11.5%), pneumonia (10.5%), and subarachnoid hemorrhage (5.5%) were the commonest individual etiologies leading to AKI. Among cardiovascular etiologies for causing AKI, emergency coronary artery bypass graft surgery, congestive heart failure, and post-coronary angiography atheroembolic disease were 3.5, 2.5, and 2%, respectively. Snakebite and organophosphorus-related AKI were seen in 3 and 1%, respectively. Tumor lysis syndrome-related AKI and multiple myeloma cast nephropathy were seen in 3.5 and 3% of patients. Among the infections, malarial AKI was seen in 1.5% of patients, and diabetic foot-cellulitis-related AKI was in 9% of cases. One patient of type 2 lepra reaction developed AKI due to rifampicin-related acute interstitial nephritis (kidney biopsy proven).
Flowchart 1.
Patients enrollment (CA-AKI and HA-AKI)
Table 1.
Patient and disease characteristics among survivors and non-survivors
| Variables | Survivors (n = 116) | Non-survivors (n = 84) | p-value (Pearson χ2/Fisher's exact test) |
|---|---|---|---|
| Comorbidities | |||
| DM, n (%) | 21 (18.1) | 37 (44.1) | 0.0001 |
| Hypertension, n (%) | 8 (6.9) | 19 (22.6) | 0.0014 |
| Cardiovascular disease, n (%) | 13 (11.2) | 24 (28.5) | 0.0019 |
| Cerebrovascular event, n (%) | 4 (3.4) | 10 (11.9) | 0.021 |
| Liver diseases, n (%) | 4 (3.4) | 11 (13.1) | 0.01 |
| Malignancy, n (%) | 4 (3.4) | 10 (11.9) | 0.02 |
| COPD, n (%) | 5 (4.3) | 4 (4.8) | 1.0 |
| Thyroid disorders, n (%) | 4 (3.4) | 2 (2.4) | 1.00 |
| Urinary system, n (%) | 8 (6.9) | 1 (1.2) | 0.08 |
| Others, n (%) | 7 (6) | 2 (2.4) | 0.30 |
| AKI stages | |||
| Stage I, n (%) | 21 (18.1) | 6 (7.1) | 0.024 |
| Stage II, n (%) | 30 (25.9) | 6 (7.1) | 0.0007 |
| Stage III, n (%) | 65 (56) | 72 (85.7) | 0.0001 |
| Age-groups | |||
| <30 years, n (%) | 17 (14.7) | 3 (3.6) | 0.008 |
| 30–60 years, n (%) | 57 (49.1) | 36 (42.9) | 0.38 |
| >60 years, n (%) | 42 (36.2) | 45 (53.6) | 0.01 |
| Etiology | |||
| Sepsis, n (%) | 13 (11.2) | 53 (63.1) | 0.0001 |
| Infections, n (%) | 51 (44) | 14 (16.7) | 0.0001 |
| Post-surgery, n (%) | 17 (14.6) | 38 (45.2) | <0.0001 |
| Cardiovascular disease, n (%) | 6 (5.2) | 1 (1.2) | 0.24 |
| Liver disease, n (%) | 4 (3.4) | 9 (10.7) | 0.04 |
| Drug induced, n (%) | 11 (9.5) | 0 | 0.003 |
| Poisoning, n (%) | 0 | 4 (4.7) | 0.018 |
| Malignancy, n (%) | 4 (3.4) | 6 (7.1) | 0.23 |
| Alcohol abuse, n (%) | 5 (4.3) | 0 | 0.075 |
| Neurological, n (%) | 7 (6) | 27 (32.1) | 0.0001 |
| Laboratory parameters | |||
| BMI median (range) | 24.6 (21.9–32.5) | 24.1 (18.1–29) | 0.15 |
| BSA median (range) | 1.85 (1.54–2.03) | 1.84 (1.51–2.04) | 0.17 |
| SOFA score median (range) | 8 (1–16) | 12 (2–18) | 0.0001 |
| Urine output median (range) | 0.4 (0.1–0.6) | 0.3 (0.1–0.6) | 0.02 |
| GFR median (range) | 36 (5–123) | 24 (4–107) | 0.004 |
| RRT | |||
| RRT requirement on admission, n (%) | 16 (13.8) | 25 (29.8) | 0.0058 |
| Mechanical ventilation | |||
| Mechanical ventilation given on admission | 18 (15.52) | 66 (78.6) | 0.0001 |
| Inotrope requirement | |||
| Inotrope not required, n (%) | 30 (25.9) | 8 (9.5) | 0.003 |
| Inotrope given = 1, n (%) | 74 (63.8) | 36 (42.9) | 0.003 |
| Inotrope given = 2, n (%) | 12 (10.3) | 32 (38.1) | <0.0001 |
| Inotrope given = 3, n (%) | 0 | 8 (9.5) | 0.0007 |
AKI, acute kidney injury; BMI, body mass index; BSA, body surface area; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; RRT, renal replacement therapy; SOFA, sequential organ failure assessment
Table 2.
Patients and disease characteristics of AKI types (CA-AKI and HA-AKI)
| Variables | CA-AKI (n = 111) | HA-AKI (n = 89) | p-value (Pearson χ2/Fisher's exact test) |
|---|---|---|---|
| Comorbid conditions | |||
| DM, n (%) | 21 (18.9) | 37 (41.6) | 0.34 |
| Hypertension, n (%) | 10 (9.0) | 17 (19.1) | 0.059 |
| Cardiovascular disease, n (%) | 11 (9.9) | 26 (29.2) | 0.0001 |
| Cerebrovascular event, n (%) | 5 (4.5) | 9 (10.1) | 0.12 |
| Liver diseases, n (%) | 8 (7.2) | 7 (7.9) | 0.009 |
| Malignancy, n (%) | 11 (9.9) | 3 (3.4) | 0.07 |
| COPD, n (%) | 8 (7.2) | 1 (1.1) | 0.045 |
| Thyroid disorders, n (%) | 2 (1.8) | 4 (4.5) | 0.41 |
| Urinary system, n (%) | 4 (3.6) | 5 (5.6) | 0.51 |
| Others, n (%) | 6 (5.4) | 3 (3.4) | 0.73 |
| Age-groups | |||
| <30 years, n (%) | 10 (9) | 10 (11.2) | 0.19 |
| 30–60 years, n (%) | 58 (52.3) | 35 (39.3) | 0.06 |
| >60 years, n (%) | 43 (38.7) | 44 (49.4) | 0.13 |
| Etiology | |||
| Sepsis, n (%) | 31 (27.9) | 35 (39.3) | 0.08 |
| Infections, n (%) | 48 (43.2) | 17 (19.1) | 0.0003 |
| Post-surgery, n (%) | 9 (8.1) | 46 (51.7) | <0.0001 |
| Cardiovascular disease, n (%) | 5 (4.5) | 2 (2.2) | 0.38 |
| Liver disease, n (%) | 13 (11.7) | 0 | 0.001 |
| Drug induced, n (%) | 9 (8.1) | 2 (2.2) | 0.11 |
| Poisoning, n (%) | 10 (9) | 0 | 0.003 |
| Malignancy, n (%) | 7 (6.3) | 0 | 0.01 |
| Alcohol abuse, n (%) | 5 (4.5) | 0 | 0.06 |
| Neurological, n (%) | 13 (11.7) | 21 (23.6) | 0.02 |
| RRT requirement (>30 days) | |||
| RRT >30 days, n (%) | 6 (5.4) | 9 (10.1) | 0.2 |
AKI, acute kidney injury; CA-AKI, community acquired AKI; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HA-AKI, hospital acquired AKI; RRT, renal replacement therapy
At the time of ICU admission, only 41 (20.5%) patients required dialysis, however, some form of dialysis was instituted in overall 95 (47.5%) patients during their ICU stay. Intermittent hemodialysis (IHD), sustained low-efficiency dialysis (SLED), continuous renal replacement therapy (CRRT), and acute peritoneal dialysis were used in 29 (30.6%), 32 (33.7%), 23 (24.3%), and 11 (11.6%) dialysis patients, respectively. Overall, 42% of patients required mechanical ventilation, with most among non-survivors (p = 0.0001), though the difference between CA-AKI and HA-AKI was insignificant (p = 0.93). About 19% of patients did not require any inotropes, 55% required a single inotrope whereas 26% of patients required ≥2 inotropes (Table 1).
Primary Outcome
The 30-day mortality was 42% and was predominantly seen among sepsis (63.1%, p = 0.0001), infections (16.7%, p = 0.0001), post-surgery (45.2%, p = <0.0001), poisoning (4.7%, p = 0.018), neurological disorders (p = 0.0001) and liver diseases (10.7%, p = 0.04). Mortality was nil in patients with drug-induced AKI (p = 0.003) and alcohol-related disorders (p = 0.075). The overall number of patients of CA-AKI and HA-AKI who required dialysis above 30 days were 5.4 and 10.1%, respectively (p = 0.21) (Table 2). Cox regression analysis revealed a higher likelihood of 30-day mortality among AKI secondary to systemic sepsis, hepatic disorders, poisoning, and neurological diseases and in those above 60 years of age (Table 3).
Table 3.
Cox regression analysis for mortality due to AKI at 30 days of hospitalization
| Variable | Walds test | Crude HR (95% CI) | p-value | Adjusted HR (95% CI) | p-value |
|---|---|---|---|---|---|
| Hypertension | 0.010 | 1.034 (0.541–1.976) | 0.920 | – | – |
| Cardiovascular disease | 0.690 | 1.421(0.620–3.257) | 0.406 | – | – |
| Malignancy | 0.530 | 0.655 (0.210–2.046) | 0.467 | – | – |
| Septicemia | 13.52 | 2.948 (1.657–5.244) | 0.0001 | 3.342 (2.035–5.489) | 0.0001 |
| Infections | 0.229 | 1.191(0.583–2.432) | 0.632 | – | – |
| Liver diseases | 9.958 | 4.065 (1.701–9.712) | 0.002 | 3.471 (1.651–7.300) | 0.001 |
| Poisoning | 28.21 | 9.784 (4.22–22.70) | 0.0001 | 9.130 (4.44–18.763) | 0.0001 |
| Neurological disease | 3.888 | 1.971 (1.004–3.869) | 0.049 | 2.126 (1.267–3.566) | 0.004 |
| Age-groups | 6.163 | 0.046 | – | – | |
| 30–60 years | 2.503 | 2.672 (0.791–9.026) | 0.114 | – | – |
| >60 years | 4.978 | 4.000 (1.18–13.51) | 0.026 | – | – |
| AKI stages | 4.443 | 0.108 | – | – | |
| AKI stage II | 0.880 | 0.560 (0.167–1.879) | 0.348 | – | – |
| AKI stage III | 0.479 | 1.385 (0.551–3.480) | 0.489 | – | – |
CI, confidence interval
Secondary Outcomes
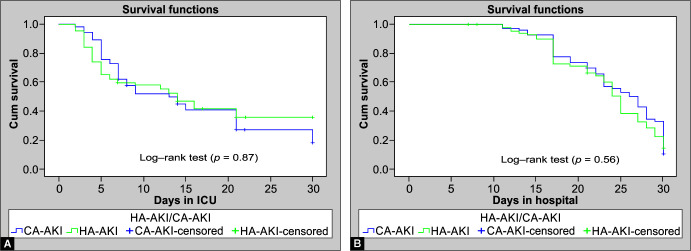
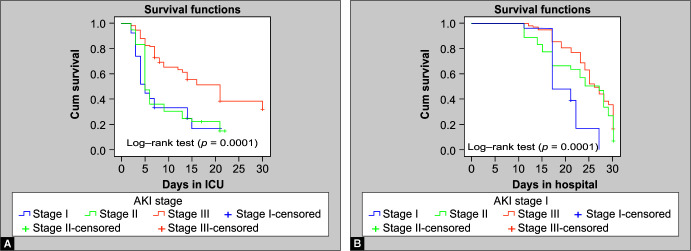
The overall median creatinine levels of the patients at the time of transfer out from the ICU and at discharge from the hospital were 3.3 and 1.2 mg/Dl, respectively. The mean eGFR (CKD-EPI) on transfer out from ICU to the general ward was 46.9 ± 18.4 mL/min/1.73 m2 and at discharge from the hospital was 67.1 ± 14.9 mL/min/1.73 m2. The median duration of stay in the ICU was 13 days for CA-AKI and 14 days for HA-AKI (p = 0.87) whereas the median stay in the hospital for CA-AKI and HA-AKI was 26 and 25 days, respectively (p = 0.56). There was no significant difference in the length of ICU stay (Fig. 1A) or hospital stay between CA-AKI and HA-AKI (Fig. 1B). The median ICU stay of KDIGO AKI stages I–III was 5 days, 5 days, and 21 days, respectively (p = 0.0001) (Fig. 2A), whereas the median hospital stays for KDIGO AKI stages I–III was 17 days, 27 days, and 25 days, respectively (p = 0.0001) (Fig. 2B). Non-survivors had significantly lower median eGFR (p = 0.004), and lower median daily urine output at the time of AKI diagnosis (p = 0.02) compared to survivors, although no such relation was seen on Cox regression analysis. However, Cox regression analysis revealed an increased likelihood of 30-day mortality among those who had a higher SOFA score at onset, lower hemoglobin, and low serum iron (Table 4).
Figs 1A and B.
Kaplan-Meier plot depicting the proportion of patients of HA-AKI and CA-AKI (y axis) in relation to (A) The length of ICU stay and (B) The length of hospital stay
Figs 2A and B.
Kaplan-Meier plot depicting the proportion of patients with KDIGO AKI stages I-III (y axis) in relation to (A) The length of ICU stay and (B) The length of hospital stay
Table 4.
Cox regression analysis of baseline continuous parameters for mortality due to AKI at 30 days of hospitalization
| Variable | Walds test | Crude HR (95% CI) | p-value | Adjusted HR (95% CI) | p-value |
|---|---|---|---|---|---|
| Urine output | 0.690 | 0.440 (0.06–3.05) | 0.406 | – | |
| eGFR | 0.541 | 1.003 (0.99–1.01) | 0.462 | – | |
| SOFA score | 6.542 | 1.097 (1.02–1.17) | 0.011 | 1.107 (1.04–1.17) | 0.001 |
| Hb | 7.371 | 0.883 (0.81–0.96) | 0.007 | 0.879 (0.81–0.96) | 0.003 |
| TLC | 1.918 | 1.00 (1.0–1.0) | 0.166 | – | |
| Iron | 9.630 | 0.983 (0.97–0.99) | 0.002 | 0.982 (0.97–0.99) | 0.001 |
| Vitamin D3 | 1.734 | 0.991 (0.97–1.01) | 0.188 | – |
CI, confidence interval; Hb, hemoglobin; eGFR, estimated glomerular filtration; TLC, total leucocyte count; SOFA, sequential organ failure assessment score
Discussion
During the COVID-19 pandemic, many ICUs were converted to COVID-19 ICUs for better management of COVID-19 and its complications including AKI all over the country. There is a paucity of published data on non-COVID-19 AKI in general ICUs during the pandemic. This study was aimed at assessing the spectrum of CA-AKI and HA-AKI, differences among survivors and non-survivors, 30-day mortality, length of ICU/hospital stay, and mortality predictors among non-COVID-19 AKI. In unison with the pre-COVID-19 (time period prior to February 2020) Indian AKI data,11–14 the majority of our population was middle-aged, although the elderly population was also reported.4 Our case mortality was higher among the older cohort and males.8,15 Mortality at 30 days was 42% in our study which was universally equatable,8,16 although the lower prevalence was seen when risk, injury, failure, loss, and end-stage kidney (RIFLE) classification was used.4 As in other studies, we had a direct relation between the AKI stage and ICU stay duration and increasing age, however, the AKI stage was not an independent factor for increased mortality.17
Sepsis-associated AKI lead the pack world over, and had worse outcomes induced drug-induced management, without any difference between CA-AKI and HA-AKI.18–20 Our study showed a significant association of sepsis with 30-day hospital mortality (adjusted HR: 3.34), thus confirming that sepsis and AKI synergistically worsen the outcomes among critically ill patients. Furthermore, our results showed less incidence of drug-induced AKI4,21 compared to a higher incidence of drug-induced HA-AKI reported by different authors.14,19 The declining trend in the incidence of drug-induced AKI may be attributed to the reduction in over-the-counter self-medication and increasing knowledge of precautions while prescribing medications, especially nephrotoxic drugs.4 The occurrence of AKI due to snake bites in our study was similar to those previously reported from India.4,12,14,18 Three patients of snake bite-induced AKI died due to a delay in hospital visits due to COVID-19 restrictions and their preference for alternative therapeutics over an urgent tertiary center visit. All of them required RRT at admission and conformed to higher mortality.22
Although DM in isolation did not have increased mortality, its association with other diseases tilted the balance. Increased mortality was seen to be associated with the presence of malignancy, but no significance was seen after adjusting the covariates.23 The odds of death were high with the presence of hematological malignancy.24 The occurrence of AKI in a general medical ward was an independent risk factor for death with hematological malignancy, use of inotropes, and higher serum creatinine in a southern India study, although this was not our mandate.24 Similarly, as in previous studies, our mortality was significantly higher in patients requiring more than one inotrope.11 Our study had an occurrence of hepatic disorders with AKI in 7.5% of patients, and the 30-day mortality was significantly high (adjusted HR 3.47), similar to other studies in the literature.25–27
Patients with multiple comorbid conditions, such as hypertension, malignancy, DM, cardiovascular diseases, cerebrovascular events, and chronic obstructive pulmonary disease (COPD), were significantly associated with mortality.4,13,19,21,28,29 In addition, the mortality among AKI patients was directly proportional to the need for mechanical ventilation and increasing inotropic requirement.8,18,28 This indirectly conformed to the higher propensity of AKI as part of multiorgan system involvement to have adverse outcomes in the best of tertiary care centers, leave alone the smaller ICUs. All the pre-COVID era studies had a varied distribution of CA-AKI and HA-AKI, mostly the latter, due to surgical AKIs.4,11,12,14,30 In comparison, our study among non-COVID-19 AKI during the COVID-19 pandemic had a predominance of CA-AKI. The reason for this difference is due to the lower number of elective surgeries being done during the current pandemic. We did not include obstetric AKI as they were managed in the obstetric ward, and we also excluded trauma patients in this study as our hospital does not primarily treat trauma cases.
The length of stay in the ICU and hospital was similar between CA-AKI and HA-AKI. Furthermore, AKI stage III had longer stays in ICU and hospital as compared to AKI stages I and II, because this subset was sicker and had multiple comorbidities, and required RRT for a prolonged period. As in other studies, AKI stage III was an independent predictor of mortality.29 The cause of AKI and timing of RRT initiation did not have any causal association with above 30 days RRT requirement.31 We utilized all modalities of RRT as described in the results section. Acute peritoneal dialysis and CRRT were done in multiorgan involvement patients with more than two inotropic requirements. Similar to studies, urine output was an important parameter for predicting mortality in AKI, and utilization of this criteria detects AKI much earlier than serum creatinine criteria and may double AKI incidences in critically ill patients.32
Anemia, leucopenia, leukocytosis, and thrombocytopenia can estimate illness severity in AKI.33 In our study, SOFA score was more predictive of adverse outcomes as published earlier for surgical ICUs.34 However no single scoring system was sufficiently sensitive and specific in predicting the development of septic AKI and in-hospital mortality for critically ill patients.34 The baseline SOFA score was a strong predictor of mortality in our study.
The limitations of our study were that it was a single-center study with a limited number of ICUs, a limited number of patients, and a lesser number of HA-AKI due to restricted elective surgeries due to the COVID-19 pandemic. We did negate many confounders by excluding patients with prior episodes of AKI, prior or current COVID-19 infection, CKD, renal transplantation, previous dialysis history, and organ donors. The strength of our study was that data was collected from more than 2 ICUs, and detailed multivariate analysis was done for clinical and biochemical parameters for the association, causation, and mortality risk calculation.
Conclusion
Early identification of patients at risk of AKI may help to implement strategies to prevent this highly lethal and morbid condition and an early referral to a critical care unit having nephrology support may prevent adverse outcomes. The risk of mortality due to AKI increases with advanced age, higher stage of AKI, the requirement of RRT, hepatic dysfunction, higher SOFA score, anemia, lower serum iron levels, and mechanical ventilation at admission. The incidence of CA-AKI was more than HA-AKI among non-COVID-19 patients due to restricted elective surgeries during the current COVID-19 pandemic.
Orcid
Bhupinder Singh https://orcid.org/0000-0002-2062-9418
Pavitra Manu Dogra https://orcid.org/0000-0002-0582-3845
Vivek Sood https://orcid.org/0000-0002-0202-6242
Vishal Singh https://orcid.org/0000-0002-8379-6395
Amit Katyal https://orcid.org/0000-0001-5180-6379
Manish Dhawan https://orcid.org/0000-0001-7537-0100
Shyam Madabhushi https://orcid.org/0000-0001-7688-1914
Krishna M Kumar https://orcid.org/0000-0002-2493-5391
Bhupendra Singh https://orcid.org/0000-0001-5807-9530
Abhishek Sharma https://orcid.org/0000-0003-0709-6298
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, et al. Acute kidney injury: An increasing global concern. Lancet. 2013;382(9887):170179. doi: 10.1016/S0140-6736(13)60647-9. [DOI] [PubMed] [Google Scholar]
- 2.Ponce D, Balbi A. Acute kidney injury: Risk factors and management challenges in developing countries. Int J Nephrol Renovasc Dis. 2016;9(1):193200. doi: 10.2147/IJNRD.S104209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coca SG, Singanamala S, Parikh CR. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012;81(5):442448. doi: 10.1038/ki.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eswarappa M, Gireesh MS, Ravi V, Kumar D, Dev G. Spectrum of acute kidney injury in critically ill patients: A single centre study from South India. Indian J Nephrol. 2014;24(5):280285. doi: 10.4103/0971-4065.132991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta RL, Cerda J, Burdmann EA, Tonelli M, García–García G, Jha V, et al. International Society of Nephrology's 0 by 25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet. 2015;385(9987):2616–2643. doi: 10.1016/S0140-6736(15)60126-X. [DOI] [PubMed] [Google Scholar]
- 6.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, et al. World incidence of AKI: A meta-analysis. Clin J Am Soc Nephrol. 2013;8(9):1482–1493. doi: 10.2215/CJN.00710113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gurjar M, Baronia AK, Azim A, Prasad N, Jain S, Singh RK, et al. Septic acute kidney injury in critically ill Indian patients. Indian J Crit Care Med. 2013;17(1):4952. doi: 10.4103/0972-5229.112147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korula S, Balakrishnan S, Sundar S, Paul V, Balagopal A. Acute kidney injury-incidence, prognostic factors, and outcome of patients in an intensive care unit in a tertiary center: A prospective observational study. Indian J Crit Care Med. 2016;20(6):332336. doi: 10.4103/0972-5229.183904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh TB, Rathore SS, Choudhury TA, Shukla VK, Singh DK, Prakash J, et al. Hospital-acquired acute kidney injury in medical, surgical, and intensive care unit: A comparative study. Indian J Nephrol. 2013;23(1):2429. doi: 10.4103/0971-4065.107192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, et al. Kidney disease: Improving global outcomes (KDIGO). Acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Internat Suppl. 2012;2(1):1–138. [Google Scholar]
- 11.Priyamvada PS, Jayasurya R, Shankar V, Parameswaran S. Epidemiology and outcomes of acute kidney injury in critically ill: Experience from a tertiary care center. Indian J Nephrol. 2018;28(6):413–420. doi: 10.4103/ijn.IJN_191_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aggarwal H, Deepak J, Ajit S, Kanwar RY, Promil J. Spectrum and outcome of acute kidney injury: A tertiary care centre experience from north India. J Ann Eu Med. 2017;5(3):53–59. [Google Scholar]
- 13.Kashinkunti MD, Dhananjaya MM. Clinical spectrum of acute kidney injury: A study from tertiary care hospital. Int J Pharmaceut Biol Res. 2013;4(4):976–979. [Google Scholar]
- 14.Kumar S, Raina S, Vikrant S, Patial RK. Spectrum of acute kidney injury in the Himalayan region. Indian J Nephrol. 2012;22(5):363–366. doi: 10.4103/0971-4065.103914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cartin–Ceba R, Kashiouris M, Plataki M, et al. Risk factors for development of acute kidney injury in critically ill patients: A systematic review and meta-analysis of observational studies. Crit Care Res Pract. 2012;2012:1–15. doi: 10.1155/2012/691013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dlamini TAL, Heering PJ, Chivese T, Rayner B. A prospective study of the demographics, management and outcome of patients with acute kidney injury in Cape Town, South Africa. PLoS One. 2017;12(6):1–12. doi: 10.1371/journal.pone.0177460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trongtrakul K, Sawawiboon C, Wang AY, Chitsomkasem A, Limphunudom P, Kurathong S, et al. Acute kidney injury in critically ill surgical patients: Epidemiology, risk factors and outcomes. Nephrology (Carlton) 2019;24(1):39–46. doi: 10.1111/nep.13192. [DOI] [PubMed] [Google Scholar]
- 18.Kaul A, Sharma RK, Tripathi R, Suresh KJ, Bhatt S, Prasad N, et al. Spectrum of community-acquired acute kidney injury in India: A retrospective study. Saudi J Kidney Dis Transpl. 2012;23(3):619–628. 22569459 [PubMed] [Google Scholar]
- 19.Goswami S, Pahwa N, Vohra R, Raju BM. Clinical spectrum of hospital acquired acute kidney injury: A prospective study from Central India. Saudi J Kidney Dis Transpl. 2018;29(4):946–955. doi: 10.4103/1319-2442.239650. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimoto K, Komaru Y, Iwagami M, Doi K. Acute kidney injury in sepsis: Evidence from Asia. Semin Nephrol. 2020;40(5):489–497. doi: 10.1016/j.semnephrol.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Vikrant S, Gupta D, Singh M. Epidemiology and outcome of acute kidney injury from a tertiary care hospital in India. Saudi J Kidney Dis Transpl. 2018;29(4):956–966. doi: 10.4103/1319-2442.239633. [DOI] [PubMed] [Google Scholar]
- 22.Vikrant S, Jaryal A, Gupta D, Parashar A. Epidemiology and outcome of acute kidney injury due to venomous animals from a subtropical region of India. Clin Toxicol (Phila) 2019;57(4):240–245. doi: 10.1080/15563650.2018.1513526. [DOI] [PubMed] [Google Scholar]
- 23.Lahoti A, Humphreys B. AKI associated with malignancies. Asn-online.org. 2016. Available at: https://www.asn-online.org/education/distancelearning/curricula/onco/Chapter3.pdf. [Google Scholar]
- 24.Chandiraseharan VK, Kalimuthu M, Prakash TV, George T, Rajenesh A, Jayaseelan V, et al. Acute kidney injury is an independent predictor of in-hospital mortality in a general medical ward: A retrospective study from a tertiary care centre in south India. Indian J Med Res. 2020;152(4):386–392. doi: 10.4103/ijmr.IJMR_1685_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daher EF, Silva GB, Santos SQ, Bezerra CC, Diniz EJB, Lima RSA, et al. Differences in community, hospital and intensive care unit-acquired acute kidney injury: Observational study in a nephrology service of a developing country. Clin Nephrol. 2012;78(6):449–455. doi: 10.5414/CN107167. [DOI] [PubMed] [Google Scholar]
- 26.Prakash J, Singh TB, Ghosh B, Malhotra V, Rathore SS, Vohra R, et al. Changing epidemiology of community-acquired acute kidney injury in developing countries: Analysis of 2405 cases in 26 years from eastern India. Clin Kidney J. 2013;6(1):150–155. doi: 10.1093/ckj/sfs178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Riordan A. Liver disease and renal dysfunction. Medicine. 2015;43(9):545–549. doi: 10.1016/j.mpmed.2015.06.004. [DOI] [Google Scholar]
- 28.Saxena A, Meshram SV. Predictors of mortality in acute kidney injury patients admitted to medicine intensive care unit in a rural tertiary care hospital. Indian J Crit Care Med. 2018;22(4):231–237. doi: 10.4103/ijccm.IJCCM_462_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teo SH, Lee KG, Koniman R, et al. A prospective study of clinical characteristics and outcomes of acute kidney injury in a tertiary care Centre. BMC Nephrol. 2019;20(1):282–290. doi: 10.1186/s12882-019-1466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L. Acute Kidney Injury in Asia. Kidney Dis (Basel) 2016;2(3):95–102. doi: 10.1159/000441887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaudry S, Hajage D, Benichou N, Chaïbi K, Barbar S, Zarbock A, et al. Delayed versus early initiation of renal replacement therapy for severe acute kidney injury: A systematic review and individual patient data meta-analysis of randomized clinical trials. Lancet. 2020;395(10235):1506–1515. doi: 10.1016/S0140-6736(20)30531-6. [DOI] [PubMed] [Google Scholar]
- 32.Koeze J, Keus F, Dieperink W, van der Horst IC, Zijlstra JG, van Meurs M. Incidence, timing and outcome of AKI in critically ill patients varies with the definition used and the addition of urine output criteria. BMC Nephrol. 2017;18(1):70–97. doi: 10.1186/s12882-017-0487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gameiro J, Lopes JA. Complete blood count in acute kidney injury prediction: A narrative review. Ann Intensive Care. 2019;9(1):87–97. doi: 10.1186/s13613-019-0561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee CW, Kou HW, Chou HS, Chou HH, Huang SF, Chang CH, et al. A combination of SOFA score and biomarkers gives a better prediction of septic AKI and in-hospital mortality in critically ill surgical patients: A pilot study. World J Emerg Surg. 2018;13(1):41–52. doi: 10.1186/s13017-018-0202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]