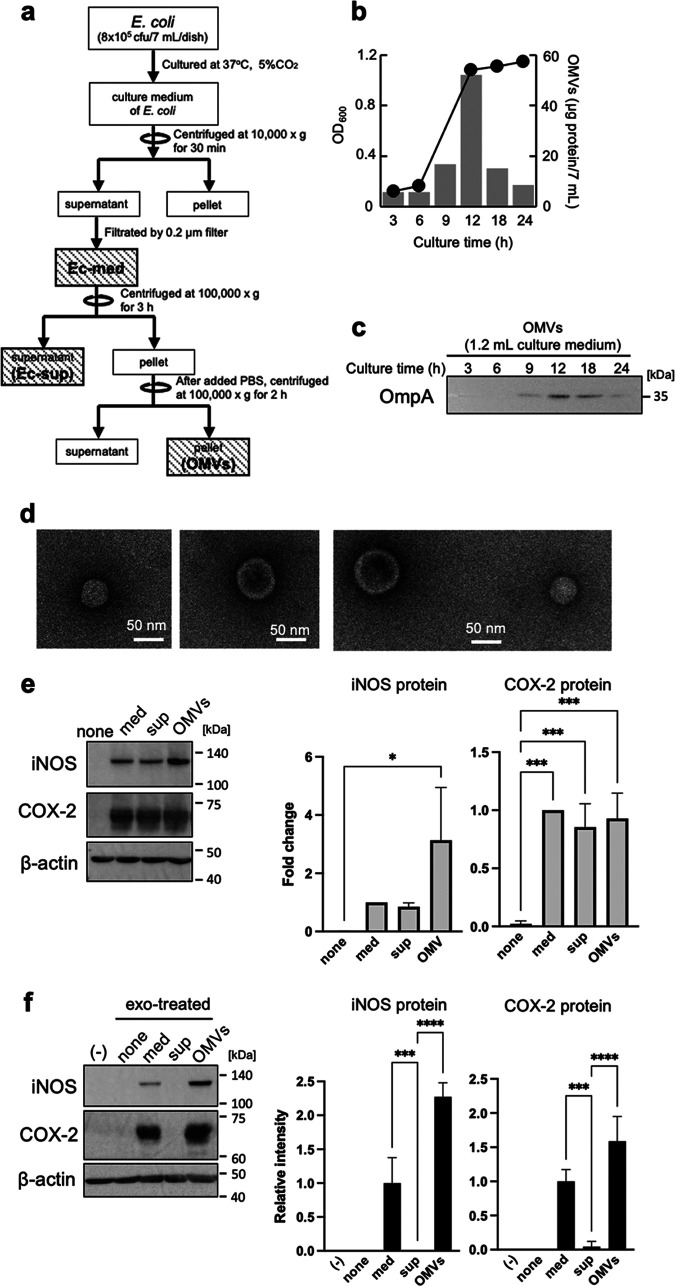
FIG 2.
Characterization of E. coli-derived outer membrane vesicles (OMVs). (a) OMVs from the culture medium of E. coli K-12 strain during 24 h were isolated by ultracentrifugation. (b) The growth of E. coli was assessed by measuring at a wavelength of 600 nm (OD600) and is shown by the line graph. The number of OMV proteins is shown in the bar graph (n = 1). The experiment was repeated with at least three independent biological replicates. (c) OMVs from 1.2 mL of culture medium were prepared by ultracentrifugation. The protein expression of OmpA, an OMV marker, was detected by Western blotting. (d) OMVs were visualized with a transmission electron microscope. (e) After RAW264.7 cells were incubated with or without Ec-med, Ec-sup, or OMVs for 9 h, cells were incubated with fresh medium for 1 h. After we changed fresh medium again, cells were incubated for 9 h. The protein expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in whole-cell lysates was measured by Western blotting. β-Actin was used as loading control. (f) After RAW264.7 cells (recipient cells; 5 × 105 cells) were incubated with each exosome from donor cells (5 × 105 cells) for 24 h, the protein expression of iNOS and COX-2 in whole-cell lysates was measured by Western blotting. β-Actin was used as loading control. The protein levels of iNOS, COX-2, and β-actin were quantitatively analyzed by using CS Analyzer 3.0 as image analysis software. Relative intensity was expressed as the mean ± SD (n = 3) of at least three independent biological replicates. One-way ANOVA and Tukey’s test were used for statistical analysis; *, P < 0.05; ***, P < 0.005; ****, P < 0.001.