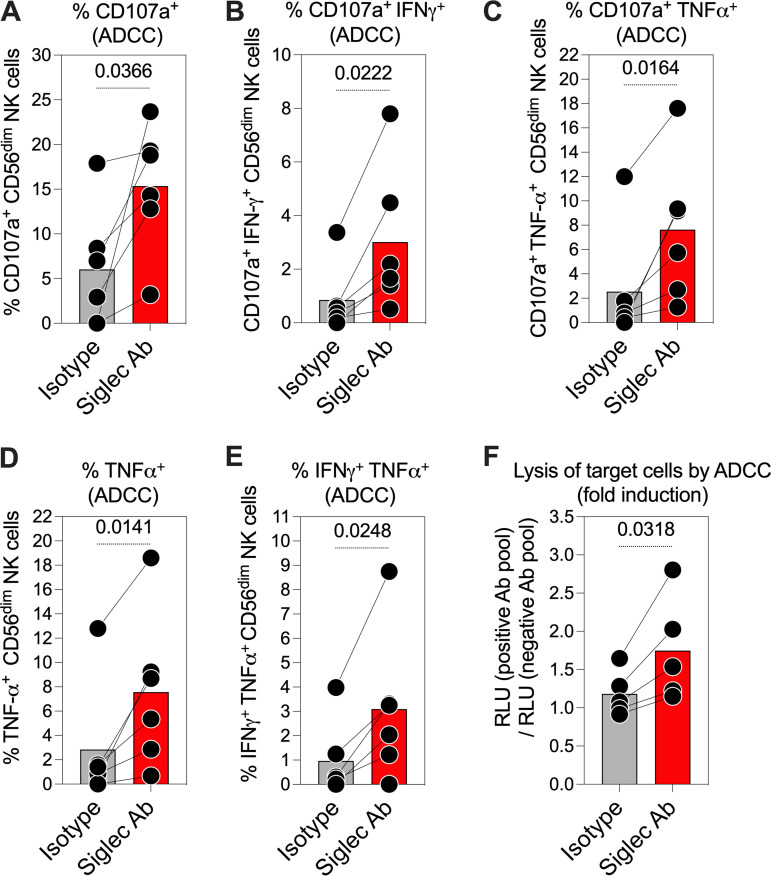
FIG 6.
Blocking Siglec-9 interactions, using a Siglec-9-blocking antibody, enhances the anti-SARS-CoV-2 ADCC of CD56dim NK cells. (A to E) The impact of the Siglec-9-blocking antibody, compared to an isotype control, on ADCC-mediated NK degranulation and/or cytokine production against SARS-CoV-2. PBMCs from six healthy controls were used as effector cells, and SARS-CoV-2 Spike-expressing 293T cells were used as target cells. Cells were cocultured at a 10:1 (E:T) ratio for 12 h. NK degranulation and/or cytokine production was assessed as the percentage of cells expressing (A) CD107a, (B) CD107a and IFN-γ, (C) CD107a and TNF-α, (D) TNF-α, (E) and IFN-γ and TNF-α. Paired t tests were used for statistical analysis. (F) The impact of the Siglec-9-blocking antibody, compared to an isotype control, on the ADCC-mediated lysis of SARS-CoV-2 target cells. Purified NK cells isolated from the PBMCs of five healthy donors were used as effector cells, and SARS-CoV-2 S CHO-K1 cells were used as target cells. Cells were cocultured at a 5:1 E:T ratio for 5 h. The SARS-CoV-2 S CHO-K1 cells stably express the SARS-CoV-2 Spike (S) protein and a HaloTag-HiBiT protein. When the target cells are lysed by ADCC, the intracellular HaloTag-HiBiT protein interacts with the extracellular detection reagent to generate a luminescence signal that can quantitatively measure the degree of target cell lysis. A paired t test was used for statistical analysis.