Background
Although physiology‐based assessment of coronary artery stenosis using instantaneous wave‐free ratio (iFR) and fractional flow reserve (FFR) are established methods of guiding coronary revascularization, its clinical outcome in long‐term deferral needs further evaluation, especially with acute coronary syndrome as a clinical presentation. The aim was to evaluate the long‐term clinical outcome of deferral of revascularization based on iFR or FFR.
Methods and Results
This is a substudy of the iFR‐SWEDEHEART (Instantaneous Wave‐Free Ratio Versus Fractional Flow Reserve in Patients With Stable Angina Pectoris or Acute Coronary Syndrome) randomized clinical trial, where patients deferred from revascularization from each study arm were selected. Nine hundred eight patients deferred from coronary revascularization with iFR (n=473) and FFR (n=435) were followed for 5 years. The national quality registry, SWEDEHEART (Swedish Web‐System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies), was used for patient data collection and clinical follow‐up. The end point was major adverse cardiac events and their individual components all‐cause death, cardiovascular death, noncardiovascular death, nonfatal myocardial infarction, and unplanned revascularization. No significant difference was found in major adverse cardiac events (iFR 18.6% versus FFR 16.8%; adjusted hazard ratio, 1.08 [95% CI, 0.79–1.48]; P=0.63) or their individual components.
Conclusions
No differences in clinical outcomes after 5‐year follow‐up were noted when comparing iFR versus FFR as methods for deferral of coronary revascularization in patients presenting with stable angina pectoris and acute coronary syndrome.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT02166736.
Keywords: acute coronary syndrome, fractional flow reserve, instantaneous wave‐free ratio, stable angina pectoris
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Revascularization
Nonstandard Abbreviations and Acronyms
- FFR
fractional flow reserve
- iFR
instantaneous wave‐free ratio
- iFR‐SWEDEHEART
Instantaneous Wave‐Free Ratio Versus Fractional Flow Reserve in Patients With Stable Angina Pectoris or Acute Coronary Syndrome
- MACE
major adverse cardiac event
- SAP
stable angina pectoris
Clinical Perspective.
What Is New?
Instantaneous wave‐free ratio and fractional flow reserve can be used as valuable methods for deferral of coronary artery stenoses in the long‐term perspective in patients presenting with stable angina pectoris and acute coronary syndrome.
What Are the Clinical Implications?
Instantaneous wave‐free ratio and fractional flow reserve can be used in clinical practice interchangeably to assess lesion severity with similar long‐term clinical outcomes.
Instantaneous wave‐free ratio (iFR) and fractional flow reserve (FFR) are established physiology‐based methods recommended in clinical guidelines for guiding coronary revascularization in intermediate‐grade stenoses. 1 The FFR guidance of coronary revascularization has been evaluated in several studies beginning in the early 1990s, and its long‐term safety has been established. 2 , 3 , 4 , 5 , 6
In 2 large randomized clinical trials, iFR‐SWEDEHEART (Instantaneous Wave‐Free Ratio Versus Fractional Flow Reserve in Patients With Stable Angina Pectoris or Acute Coronary Syndrome) and DEFINE‐FLAIR (Functional Lesion Assessment of Intermediate Stenosis to Guide Revascularization), the resting index iFR was found to be noninferior to FFR in guiding coronary revascularization with respect to clinical outcome at 1 year. 7 , 8 Although iFR had previously been validated in several small studies, 9 , 10 , 11 , 12 the results of the cited randomized studies provided evidence to support its use to guide coronary revascularization. Combining the populations of the 2 studies 7 , 8 provided a unique opportunity to investigate the deferral of revascularization based on iFR and FFR using current cutoff values and medical treatment. Results from the pooled population revealed no difference between iFR and FFR in the rate of major adverse cardiac events (MACEs) at 1 year among patients who were deferred from revascularization. 13 The observed event rate among patients presenting with acute coronary syndrome (ACS) was significantly higher than that of patients presenting with stable angina pectoris (SAP).
Recently published 5‐year results from the iFR‐SWEDEHEART trial confirm the long‐term safety of iFR‐guided revascularization. 14 However, this also provided the opportunity to evaluate deferral of revascularization long term in both iFR and FFR in a large patient population, making it one of the largest long‐term deferral studies in the field.
The objective of this study was to investigate the 5‐year outcome of patients deferred from revascularization in the iFR‐SWEDEHEART trial. We investigated the long‐term clinical outcomes of deferral with iFR and FFR as well as clinical outcomes by clinical presentation.
METHODS
Study Design of the IFR‐SWEDEHEART Trial
iFR‐SWEDEHEART was a multicenter, randomized controlled, open‐label clinical trial. 7 A national quality registry, SWEDEHEART (Swedish Web‐System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies), was accessed for patient data collection, randomization, and clinical follow‐up from year 1 through year 5. The trial was conducted in compliance with the Declaration of Helsinki criteria and approved by the ethical review boards of Sweden, Denmark, and Iceland. It was funded by an unrestricted research grant from the Volcano Corporation, which had no influence over the study design, analysis, or reporting of results.
All patients were included in the web‐based SCAAR (Swedish Coronary Angiography and Angioplasty Registry) platform, a component of the SWEDEHEART registry. The SCAAR contains data from a single catheterization laboratory in Iceland and all 30 catheterization laboratory centers in Sweden. A center in Denmark participated, entering all relevant data from the Western Denmark Heart Registry into SCAAR. After baseline information was entered, patients were randomized to undergo revascularization guided by either iFR or FFR. Relevant follow‐up data were obtained from the national registries.
Study Design of the Present Study
The present study is a substudy of clinical outcomes of the deferred population from the iFR‐SWEDEHEART trial at 5‐year follow‐up. This means no revascularizations were performed on patients included in this study. A subgroup analysis comparing clinical presentation of ACS and SAP was included. The authors declare that all supporting data are available within the article.
Study Population
The study population comprised patients with SAP and ACS, including unstable angina pectoris and non–ST‐segment–elevation myocardial infarction. The patients exhibited indications for physiologically guided assessment of at least 1 coronary lesion as determined by the operator (lesions with 40%–80% stenosis by visual estimate). In patients with SAP, any lesion could be assessed, whereas in those with ACS, only nonculprit lesions were assessed using physiology‐based techniques. Major exclusion criteria were known terminal disease with a life expectancy of <1 year, unstable hemodynamic, inability to tolerate adenosine, previous coronary artery bypass grafting with patent graft to the interrogated vessel, heavily calcified or tortuous vessel in which ability to cross the lesion with a pressure wire was unlikely, inability to provide informed consent, and previous randomization in the iFR‐SWEDEHEART trial. All participants provided written informed consent before enrolment.
Procedure
A coronary‐pressure guidewire (Philips Volcano) was used for measurement of iFR and FFR. Intracoronary nitroglycerin was administered before the procedure to prevent any coronary spasms that would otherwise affect the measurements. Hyperemia during FFR was induced with adenosine either as an intracoronary bolus injection or as an intravenous infusion until stable hyperemia was obtained. The thresholds for indication of a hemodynamically important stenosis were prespecified as 0.89 for iFR and 0.80 for FFR. Treatment was deferred with values over these thresholds. The physician, according to clinical practice, determined the pharmacological treatment.
End Points
The primary end point was the rate of MACEs, defined as all‐cause death, nonfatal myocardial infarction (MI), or unplanned revascularization at 5 years. Secondary end points included MACEs and their individual components. The patients' unique Swedish personal identification numbers were linked to the Swedish National Population Registry by the Epidemiologic Center of the Swedish National Board of Health and Welfare to obtain information of mortality. Information about nonfatal MI and unplanned revascularization were obtained from SWEDEHEART. The definition of unplanned revascularization was revascularization by either percutaneous coronary intervention or coronary artery bypass grafting that was not anticipated at the index procedure and not a part of a staged procedure to be performed within 60 days.
Statistical Analysis
Categorical data are presented as counts and percentages and tested with χ2. Continuous variables are presented as mean±SD and tested with a 2‐tailed Student t test. Missing values constituted <2% of data and were ignored in all analyses. The end point of MACEs and their individual components of all‐cause death, cardiovascular death, noncardiovascular death, nonfatal MI, and unplanned revascularization were analyzed on a per‐protocol basis. A time‐to‐event analysis was performed by Cox proportional hazard models. The validity of the proportional hazard assumption was tested. Results are presented with hazard ratio (HR) and 2‐sided 95% CI. Results were analyzed unadjusted as well as adjusted for age, sex, body mass index, diabetes, hypertension, hyperlipidemia, smoking status, previous MI, previous percutaneous coronary intervention, and clinical presentation. A 2‐sided P value <0.05 was considered statistically significant. Kaplan‐Meier survival curves were plotted for visual comparison of iFR and FFR groups and clinical presentation. All statistical analyses were performed using Stata version 17 (StataCorp).
RESULTS
Study Population
From May 2014 through October 2015, 2037 patients were randomized to undergo either iFR‐ or FFR‐guided revascularization (Figure 1). A total of 2019 patients were included in the final analysis of the original randomized iFR‐SWEDEHEART trial after exclusion of 18 patients because of technical issues, incorrect group assignment, side effects of adenosine, other medical conditions, or other reasons. Revascularization was deferred in all investigated vessels in 908 patients, 473 patients (52%) with iFR and 435 (48%) with FFR. Six hundred eleven patients presented with SAP, and 297 patients with ACS.
Figure 1. Flowchart of study design.
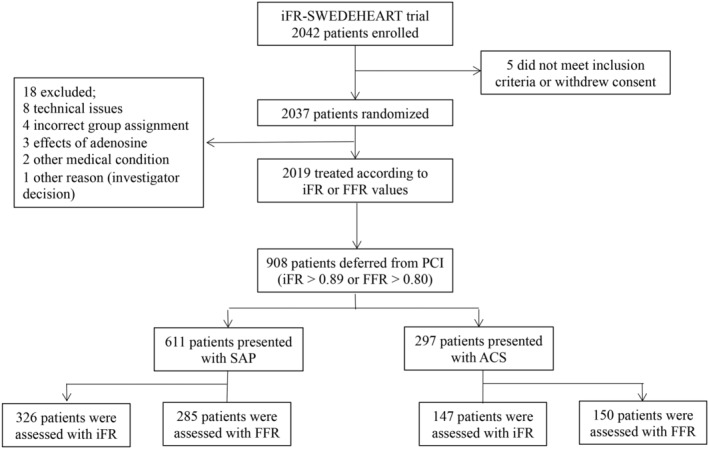
A total of 2042 patients were enrolled in the iFR‐SWEDEHEART (Instantaneous Wave‐Free Ratio Versus Fractional Flow Reserve in Patients With Stable Angina Pectoris or Acute Coronary Syndrome) randomized clinical trial, with 2019 patients included for the final analysis and 908 patients deferred from coronary revascularization. ACS indicates acute coronary syndrome; FFR, fractional flow reserve; iFR, instantaneous wave‐free ratio; PCI, percutaneous coronary intervention; and SAP, stable angina pectoris.
Baseline and Procedural Characteristics
Patient baseline characteristics are presented in Table 1. There was a significant difference in Canadian Cardiovascular Society grade of angina in the iFR group (class I, 34.7%; class II, 62.5%; class III, 2.5%) and FFR group (class I, 29.3%; class II, 59.0%; class III, 10.9%; P<0.05). The 2 groups were well balanced in the remaining baseline characteristics.
Table 1.
Baseline Characteristics
| Characteristic | iFR, n=473 | FFR, n=435 | P value |
|---|---|---|---|
| Age, y, M±SD | 67.4±9.7 | 67.2±9.3 | 0.80 |
| Men, % (n) | 68.3 (323) | 67.4 (293) | 0.76 |
| Body mass index, kg/m2, M±SD | 27.4±4.3 | 27.5±4.5 | 0.74 |
| Diabetes, % (n) | 17.6 (83) | 17.5 (76) | 0.36 |
| Hypertension, % (n) | 72.5 (342) | 69.7 (303) | 0.23 |
| Hyperlipidemia, % (n) | 74.4 (351) | 68.7 (299) | 0.10 |
| Nonsmoker, % (n) | 33.6 (159) | 36.6 (159) | <0.05 |
| Current smoker, % (n) | 16.1 (76) | 15.6 (68) | |
| Previous smoker, % (n) | 49.9 (236) | 45.7 (199) | |
| Previous MI, % (n) | 33.1 (156) | 33.1 (144) | 0.16 |
| Previous PCI, % (n) | 46.3 (219) | 43.5 (189) | 0.39 |
| CCS angina class | <0.05 | ||
| I, % (n) | 34.7 (96) | 29.3 (67) | |
| II, % (n) | 62.5 (173) | 59.0 (135) | |
| III, % (n) | 2.5 (7) | 10.9 (25) | |
| IV, % | 0 | 0 | |
| Clinical presentation | 0.28 | ||
| Acute coronary syndrome, % (n) | 31.1 (147) | 34.5 (150) | |
| Stable angina pectoris, % (n) | 68.9 (326) | 65.5 (285) |
CCS indicates Canadian Cardiovascular Society; FFR, fractional flow reserve; iFR, instantaneous wave‐free ratio; M, mean; MI, myocardial infarction; and PCI, percutaneous coronary intervention.
End Point and Components
At 5 years, the rate of MACEs was 18.6% in the iFR group and 16.8% in the FFR group (adjusted HR, 1.08 [95% CI, 0.79–1.48]; P=0.63; Figure 2). There were no significant differences in all‐cause death (iFR 7.6% versus FFR 6.7%, P=0.62), cardiovascular death (iFR 1.9% versus FFR 2.0%, P=0.94), noncardiovascular death (iFR 5.7% versus FFR 4.6%, P=0.52), nonfatal MI (iFR 5.3% versus FFR 5.5%, P=0.80), or unplanned revascularization (iFR 9.7% versus FFR 9.2%, P=0.89; Table 2). Unplanned revascularization was the most frequent component of MACEs in both groups.
Figure 2. Kaplan‐Meier curve for the cumulative risk of a MACE in deferral based on iFR and FFR.
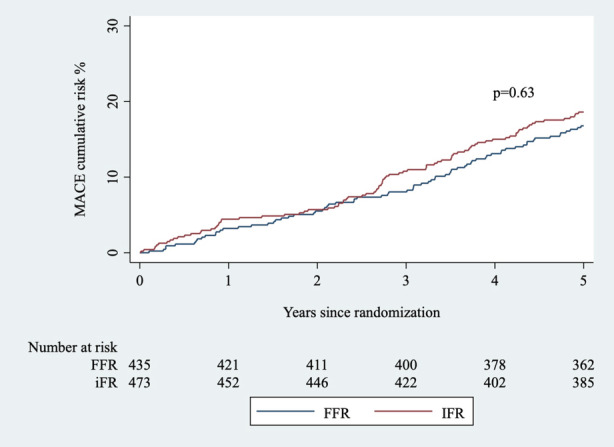
Cumulative risk of a major adverse cardiac event at 5 years with respect to method. FFR indicates fractional flow reserve; iFR, instantaneous wave‐free ratio; and MACE, major adverse cardiac event.
Table 2.
Major Adverse Cardiac Event and Its Individual Components Relative to Deferral Based on iFR and FFR
| MACE/component | iFR, n=473 | FFR, n=435 | iFR vs FFR | P value | |
|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | ||||
| MACE | 18.6 (88) | 16.8 (73) | 1.13 (0.83–1.54) | 1.08 (0.79–1.48) | 0.63 |
| All‐cause death | 7.6 (36) | 6.7 (29) | 1.14 (0.70–1.86) | 1.14 (0.69–1.87) | 0.62 |
| Cardiovascular death | 1.9 (9) | 2.0 (9) | 0.92 (0.36–2.31) | 0.97 (0.38–2.49) | 0.94 |
| Noncardiovascular death | 5.7 (27) | 4.6 (20) | 1.24 (0.70–2.22) | 1.21 (0.67–2.20) | 0.52 |
| Nonfatal MI | 5.3 (25) | 5.5 (24) | 0.97 (0.55–1.69) | 0.93 (0.58–1.63) | 0.80 |
| Unplanned revascularization | 9.7 (46) | 9.2 (40) | 1.06 (0.70–1.62) | 1.03 (0.67–1.59) | 0.89 |
Values are % (n) unless indicated otherwise. FFR indicates fractional flow reserve; HR, hazard ratio; iFR, instantaneous wave‐free ratio; MACE, major adverse cardiac event; and MI, myocardial infarction.
When outcome at 5 years was adjusted by clinical presentation, there was no difference in the composite end point of MACEs (SAP 16.7% versus ACS 19.9%; adjusted HR, 0.85 [95% CI, 0.61–1.19]; P=0.36). The HRs for the individual components of MACE adjusted by clinical presentation were not significant (Table 3). Neither the MACEs rates (Figure 3) nor their individual components were influenced by iFR versus FFR (Table 4), with a nonsignificant P value for interaction.
Table 3.
Major Adverse Cardiac Event and Its Individual Components for SAP and ACS
| MACE/component | SAP, n=611 | ACS, n=297 | SAP vs ACS | P value | |
|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | Adjusted HR (95% CI) | ||||
| MACE | 16.7 (102) | 19.9 (59) | 0.82 (0.60–1.13) | 0.85 (0.61–1.19) | 0.35 |
| All‐cause death | 6.7 (41) | 8.1 (24) | 0.82 (0.49–1.36) | 0.86 (0.51–1.44) | 0.57 |
| Cardiovascular death | 1.6 (10) | 2.7 (8) | 0.60 (0.24–1.52) | 0.60 (0.23–1.57) | 0.30 |
| Noncardiovascular death | 5.1 (31) | 5.4 (16) | 0.93 (0.51–1.70) | 0.97 (0.52–1.80) | 0.92 |
| Nonfatal MI | 5.2 (32) | 5.7 (17) | 0.91 (0.51–1.65) | 0.96 (0.53–1.74) | 0.89 |
| Unplanned revascularization | 9.3 (57) | 9.8 (29) | 0.95 (0.61–1.49) | 0.96 (0.60–1.52) | 0.85 |
Values are % (n) unless indicated otherwise. ACS indicates acute coronary syndrome; HR, hazard ratio; MACE, major adverse cardiac event; MI, myocardial infarction; and SAP, stable angina pectoris.
Figure 3. Kaplan‐Meier curve for the cumulative risk of a MACE by clinical presentation with respect to method.
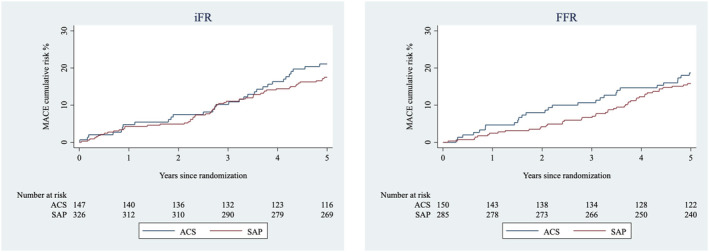
The cumulative risk of major adverse cardiac event at 5 years relative to clinical presentation (SAP and ACS) deferred based on iFR (left) and FFR (right). ACS indicates acute coronary syndrome; FFR, fractional flow reserve; iFR, instantaneous wave‐free ratio; MACE, major adverse cardiac event; and SAP, stable angina pectoris.
Table 4.
Outcomes According to Clinical Presentation and Physiology‐Based Technique
| Outcome | SAP, n=611 | ACS, n=297 | SAP vs ACS unadjusted HR (95% CI) | P value | Interaction P value |
|---|---|---|---|---|---|
| MACE | 16.7 (102) | 19.9 (59) | 0.82 (0.60–1.13) | 0.23 | |
| iFR | 17.5 (57) | 21.1 (31) | 0.82 (0.53–1.27) | 0.99 | |
| FFR | 15.8 (45) | 18.7 (28) | 0.82 (0.51–1.31) | ||
| All‐cause death | 6.7 (41) | 8.1 (24) | 0.82 (0.49–1.36) | 0.44 | |
| iFR | 7.7 (25) | 7.5 (11) | 1.03 (0.51–2.10) | 0.34 | |
| FFR | 5.6 (16) | 8.7 (13) | 0.63 (0.30–1.30) | ||
| Cardiovascular death | 1.6 (10) | 2.7 (8) | 0.60 (0.24–1.52) | 0.28 | |
| iFR | 1.8 (6) | 2.0 (3) | 0.91 (0.23–3.63) | 0.41 | |
| FFR | 1.4 (4) | 3.3 (5) | 0.41 (0.11–1.52) | ||
| Noncardiovascular death | 5.1 (31) | 5.4 (16) | 0.93 (0.51–1.70) | 0.81 | |
| iFR | 5.8 (19) | 5.4 (8) | 1.08 (0.47–2.46) | 0.58 | |
| FFR | 4.2 (12) | 5.3 (8) | 0.76 (0.31–1.87) | ||
| Nonfatal MI | 5.2 (32) | 5.7 (17) | 0.91 (0.51–1.65) | 0.77 | |
| iFR | 5.2 (17) | 5.4 (8) | 0.96 (0.41–2.22) | 0.88 | |
| FFR | 5.3 (15) | 6.0 (9) | 0.88 (0.38–2.0) | ||
| Unplanned revascularization | 9.3 (57) | 9.8 (29) | 0.95 (0.61–1.49) | 0.84 | |
| iFR | 9.2 (30) | 10.9 (16) | 0.84 (0.46–1.54) | 0.56 | |
| FFR | 9.8 (27) | 8.7 (13) | 1.10 (0.57–2.13) |
Values are % (n) unless indicated otherwise. ACS indicates acute coronary syndrome; FFR, fractional flow reserve; HR, hazard ratio; iFR, instantaneous wave‐free ratio; MACE, major adverse cardiac event; MI, myocardial infarction; and SAP, stable angina pectoris.
DISCUSSION
This substudy of the iFR‐SWEDEHEART trial, which to our knowledge constitutes the largest published study to date on long‐term deferral with iFR and FFR, demonstrated no statistically significant difference in long‐term (5 years) clinical outcome in patients deferred from coronary revascularization based on iFR and FFR. Interestingly, there did not appear to be any differences in long‐term event rates between patients presenting with SAP compared with ACS. This suggests that iFR or FFR can be used regardless of clinical presentation. However, these results should be interpreted with caution and need further evaluation, considering the wide confidence intervals and the study being a subgroup analysis.
Comparison With Previous Studies
Both iFR and FFR are well‐recognized methods for guiding coronary revascularization in intermediate coronary artery stenoses. 1 FFR was the first physiology‐based technique to be evaluated as a generalizable tool for guiding revascularization. In the DEFER (Deferral Versus Performance of Percutaneous Transluminal Coronary Angioplasty in Patients Without Documented Ischemia) study, 91 of 325 patients with FFR ≥0.75 were deferred from revascularization. 6 Follow‐up for as long as 15 years has confirmed its safety. 2 , 15 Comparison of these results with those of current standard procedure is difficult because of changes in cutoff value recommendations together with the evolution of drug‐eluting stents and medical treatment. When the FAME (Fractional Flow Reserve Versus Angiography for Guiding Percutaneous Coronary Intervention) study was conducted, the cutoff value was similar to current guidelines, and drug‐eluting stents were used for percutaneous coronary intervention, 5 making the results more comparable with our study, although guideline recommendations for use of coronary physiology‐based assessment differ from when the FAME study was conducted. Of the 1005 patients included in the FAME study, 509 were assigned for treatment according to FFR values, whereas the remaining were treated based on angiography alone. At 5 years, the MACEs rate in the FFR group was 28%, similar to that of the angiography group (31%). 3 The MACEs rate of the current substudy is lower than that in the randomized FAME study. The difference could be partly attributed to modern stents and medical treatment in combination with a more efficient health care system. In addition, the population of the FAME study had more comorbidities, and inclusion criteria included multivessel disease. However, even taking those discrepancies into account, our lower event rates suggest that iFR and FFR could be used as reliable methods for deferral of coronary revascularization in the long term. A randomized study focusing on clinical outcome on a vessel level would be valuable to further deepen the knowledge about deferral with iFR and FFR.
The iFR‐SWEDEHEART and DEFINE‐FLAIR trials demonstrated the safety of coronary revascularization guided by iFR compared with FFR. 7 , 8 Its long‐term safety was confirmed in the 5‐year follow‐up of the iFR‐SWEDEHEART trial. 14 Both trials identified fewer hemodynamically significant stenoses with iFR and, consequently, a higher rate of deferral of revascularization with iFR. There was no significant difference in the rate of unplanned revascularization between iFR and FFR. The studies provided the opportunity to evaluate deferral of revascularization with both iFR and FFR in a large population. 13 The lower event rates of approximately 4% in the pooled population of the iFR‐SWEDEHEART trial and DEFINE‐FLAIR trial compared with those found in the DEFER trial (8%) could reflect the evolution of coronary revascularization in combination with medical therapy. The continued monitoring of the deferred population is essential, especially when techniques such as iFR identify fewer stenoses as hemodynamically significant. In our long‐term substudy follow‐up of deferral from the iFR‐SWEDEHEART trial, there appeared to be no difference in the rate of MACEs between the iFR and FFR groups. Thus, the favorable results seen at 1 year follow‐up were preserved in this study, suggesting that there may not be a difference in clinical outcome with deferral using either iFR or FFR on a patient level. The event rate in the FFR group (16.8%) at 5 years remained lower than that reported in the DEFER trial (21%). 15 However, the event rate was not as low as might be expected when compared with the 2‐fold difference at 1 year between the pooled population and the DEFER trial. Our study included patients presenting with SAP and ACS. Over time, patients with ACS exhibit higher cardiovascular risk potentially influencing the results. The patients in our study were older than the patients in the DEFER study, which may explain the relatively higher rate of noncardiovascular deaths.
Deferral of Revascularization Relative to Clinical Presentation
The use of FFR to guide coronary revascularization in patients with SAP is well established and considered safe. 4 , 5 Reports of its safety in patients presenting with ACS are inconsistent. Two randomized clinical trials evaluating patients presenting with ST‐segment–elevation myocardial infarction showed that FFR‐guided complete revascularization of nonculprit lesions to reduce the risk of a composite cardiovascular outcome was mainly driven by revascularization. 16 , 17 The primary concern of using FFR in patients with ACS is that microvascular circulation is disturbed in the acute phase; however, studies support the use of FFR in this situation in nonculprit lesions. 18 A limitation to those studies is that they focused on patients presenting with ACS and did not compare results with patients presenting with SAP.
In the pooled analysis of the iFR‐SWEDEHEART and DEFINE‐FLAIR trials with patients deferred from revascularization with iFR and FFR, the 1‐year rate of MACEs was significantly higher in patients presenting with ACS compared with patients with SAP. 13 These results are in agreement with other studies demonstrating that FFR‐guided revascularization is associated with a higher rate of MACEs in patients with ACS compared with those with SAP. 19 , 20 , 21 However, on the matter of deferral, the present study found no significant difference in MACE or its individual components after 5 years when patients were deferred with iFR and FFR, regardless of clinical presentation. These results should be interpreted with caution because of the substudy design of the trial, this being a subgroup analysis within a substudy, and the wide range of confidence intervals. Further investigations are needed in this field also focusing on clinical presentation on a vessel level.
Limitations
This was a substudy of the randomized iFR‐SWEDEHEART trial, where patients deferred from iFR and FFR were followed over time. The results were fully adjusted for baseline characteristics. However, the observational nature of this post hoc study means that residual confounding cannot be ruled out. The iFR‐SWEDEHEART trial was not powered for this specific subgroup analysis with a risk for type I error. There is a possibility for a concealed difference in clinical presentation given the wide confidence intervals and this being a subgroup analysis within a substudy. The end point of MACEs was not specified on a vessel level, and we could not differentiate between target and nontarget vessel outcomes. Therefore, the clinical outcome could potentially be driven by new events that were not related to the deferred lesion.
CONCLUSIONS
No differences in clinical outcomes after 5 years were noted when comparing iFR versus FFR as methods for deferral of coronary revascularization in patients presenting with SAP and ACS.
Sources of Funding
This work was supported by Philips Healthcare.
Disclosures
Dr Götberg is the European principal investigator for Distal Evaluation of Functional Performance With Intravascular Sensors to Assess the Narrowing Effect: Guided Physiologic Stenting (DEFINE‐GPS) and retains minor consulting honoraria by Philips in relation to study activities. The remaining authors have no disclosures to report.
For Sources of Funding and Disclosures, see page 8.
References
- 1. Neumann FJ, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, et al. ESC/EACTS Guidelines on myocardial revascularization. EuroIntervention. 2018;2019:1435–1534. doi: 10.4244/EIJY19M01_01 [DOI] [PubMed] [Google Scholar]
- 2. Zimmermann FM, Ferrara A, Johnson NP, van Nunen LX, Escaned J, Albertsson P, Erbel R, Legrand V, Gwon HC, Remkes WS, et al. Deferral vs. performance of percutaneous coronary intervention of functionally non‐significant coronary stenosis: 15‐year follow‐up of the DEFER trial. Eur Heart J. 2015;36:3182–3188. doi: 10.1093/eurheartj/ehv452 [DOI] [PubMed] [Google Scholar]
- 3. van Nunen LX, Zimmermann FM, Tonino PA, Barbato E, Baumbach A, Engstrom T, Klauss V, MacCarthy PA, Manoharan G, Oldroyd KG, et al. Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME): 5‐year follow‐up of a randomised controlled trial. Lancet. 2015;386:1853–1860. doi: 10.1016/S0140-6736(15)00057-4 [DOI] [PubMed] [Google Scholar]
- 4. De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Mobius‐Winkler S, Rioufol G, Witt N, et al. Fractional flow reserve‐guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. doi: 10.1056/NEJMoa1205361 [DOI] [PubMed] [Google Scholar]
- 5. Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van't Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611 [DOI] [PubMed] [Google Scholar]
- 6. Bech GJ, De Bruyne B, Pijls NH, de Muinck ED, Hoorntje JC, Escaned J, Stella PR, Boersma E, Bartunek J, Koolen JJ, et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation. 2001;103:2928–2934. doi: 10.1161/01.CIR.103.24.2928 [DOI] [PubMed] [Google Scholar]
- 7. Gotberg M, Christiansen EH, Gudmundsdottir IJ, Sandhall L, Danielewicz M, Jakobsen L, Olsson SE, Ohagen P, Olsson H, Omerovic E, et al. Instantaneous wave‐free ratio versus fractional flow reserve to guide PCI. N Engl J Med. 2017;376:1813–1823. doi: 10.1056/NEJMoa1616540 [DOI] [PubMed] [Google Scholar]
- 8. Davies JE, Sen S, Dehbi HM, Al‐Lamee R, Petraco R, Nijjer SS, Bhindi R, Lehman SJ, Walters D, Sapontis J, et al. Use of the instantaneous wave‐free ratio or fractional flow reserve in PCI. N Engl J Med. 2017;376:1824–1834. doi: 10.1056/NEJMoa1700445 [DOI] [PubMed] [Google Scholar]
- 9. Petraco R, van de Hoef TP, Nijjer S, Sen S, van Lavieren MA, Foale RA, Meuwissen M, Broyd C, Echavarria‐Pinto M, Foin N, et al. Baseline instantaneous wave‐free ratio as a pressure‐only estimation of underlying coronary flow reserve: results of the JUSTIFY‐CFR study (Joined Coronary Pressure and Flow Analysis to Determine Diagnostic Characteristics of Basal and Hyperemic Indices of Functional Lesion Severity‐Coronary Flow Reserve). Circ Cardiovasc Interv. 2014;7:492–502. doi: 10.1161/CIRCINTERVENTIONS.113.000926 [DOI] [PubMed] [Google Scholar]
- 10. Sen S, Asrress KN, Nijjer S, Petraco R, Malik IS, Foale RA, Mikhail GW, Foin N, Broyd C, Hadjiloizou N, et al. Diagnostic classification of the instantaneous wave‐free ratio is equivalent to fractional flow reserve and is not improved with adenosine administration. Results of CLARIFY (Classification Accuracy of Pressure‐Only Ratios Against Indices Using Flow Study). J Am Coll Cardiol. 2013;61:1409–1420. doi: 10.1016/j.jacc.2013.01.034 [DOI] [PubMed] [Google Scholar]
- 11. Petraco R, Escaned J, Sen S, Nijjer S, Asrress KN, Echavarria‐Pinto M, Lockie T, Khawaja MZ, Cuevas C, Foin N, et al. Classification performance of instantaneous wave‐free ratio (iFR) and fractional flow reserve in a clinical population of intermediate coronary stenoses: results of the ADVISE registry. EuroIntervention. 2013;9:91–101. doi: 10.4244/EIJV9I1A14 [DOI] [PubMed] [Google Scholar]
- 12. Sen S, Escaned J, Malik IS, Mikhail GW, Foale RA, Mila R, Tarkin J, Petraco R, Broyd C, Jabbour R, et al. Development and validation of a new adenosine‐independent index of stenosis severity from coronary wave‐intensity analysis: results of the ADVISE (ADenosine Vasodilator Independent Stenosis Evaluation) study. J Am Coll Cardiol. 2012;59:1392–1402. doi: 10.1016/j.jacc.2011.11.003 [DOI] [PubMed] [Google Scholar]
- 13. Escaned J, Ryan N, Mejia‐Renteria H, Cook CM, Dehbi HM, Alegria‐Barrero E, Alghamdi A, Al‐Lamee R, Altman J, Ambrosia A, et al. Safety of the deferral of coronary revascularization on the basis of instantaneous wave‐free ratio and fractional flow reserve measurements in stable coronary artery disease and acute coronary syndromes. JACC Cardiovasc Interv. 2018;11:1437–1449. doi: 10.1016/j.jcin.2018.05.029 [DOI] [PubMed] [Google Scholar]
- 14. Gotberg M, Berntorp K, Rylance R, Christiansen EH, Yndigegn T, Gudmundsdottir IJ, Koul S, Sandhall L, Danielewicz M, Jakobsen L, et al. 5‐year outcomes of PCI guided by measurement of instantaneous wave‐free ratio versus fractional flow reserve. J Am Coll Cardiol. 2022;79:965–974. doi: 10.1016/j.jacc.2021.12.030 [DOI] [PubMed] [Google Scholar]
- 15. Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van't Veer M, Bar F, Hoorntje J, Koolen J, Wijns W, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5‐year follow‐up of the DEFER study. J Am Coll Cardiol. 2007;49:2105–2111. doi: 10.1016/j.jacc.2007.01.087 [DOI] [PubMed] [Google Scholar]
- 16. Engstrom T, Kelbaek H, Helqvist S, Hofsten DE, Klovgaard L, Holmvang L, Jorgensen E, Pedersen F, Saunamaki K, Clemmensen P, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST‐segment elevation myocardial infarction and multivessel disease (DANAMI‐3‐PRIMULTI): an open‐label, randomised controlled trial. Lancet. 2015;386:665–671. doi: 10.1016/S0140-6736(15)60648-1 [DOI] [PubMed] [Google Scholar]
- 17. Smits PC, Abdel‐Wahab M, Neumann FJ, Boxma‐de Klerk BM, Lunde K, Schotborgh CE, Piroth Z, Horak D, Wlodarczak A, Ong PJ, et al. Fractional flow reserve‐guided multivessel angioplasty in myocardial infarction. N Engl J Med. 2017;376:1234–1244. doi: 10.1056/NEJMoa1701067 [DOI] [PubMed] [Google Scholar]
- 18. Ntalianis A, Sels JW, Davidavicius G, Tanaka N, Muller O, Trana C, Barbato E, Hamilos M, Mangiacapra F, Heyndrickx GR, et al. Fractional flow reserve for the assessment of nonculprit coronary artery stenoses in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2010;3:1274–1281. doi: 10.1016/j.jcin.2010.08.025 [DOI] [PubMed] [Google Scholar]
- 19. Hakeem A, Edupuganti MM, Almomani A, Pothineni NV, Payne J, Abualsuod AM, Bhatti S, Ahmed Z, Uretsky BF. Long‐term prognosis of deferred acute coronary syndrome lesions based on nonischemic fractional flow reserve. J Am Coll Cardiol. 2016;68:1181–1191. doi: 10.1016/j.jacc.2016.06.035 [DOI] [PubMed] [Google Scholar]
- 20. Lee JM, Choi KH, Koo BK, Shin ES, Nam CW, Doh JH, Hwang D, Park J, Zhang J, Lim HS, et al. Prognosis of deferred non‐culprit lesions according to fractional flow reserve in patients with acute coronary syndrome. EuroIntervention. 2017;13:e1112–e1119. doi: 10.4244/EIJ-D-17-00110 [DOI] [PubMed] [Google Scholar]
- 21. Masrani Mehta S, Depta JP, Novak E, Patel JS, Patel Y, Raymer D, Facey G, Zajarias A, Lasala JM, Singh J, et al. Association of lower fractional flow reserve values with higher risk of adverse cardiac events for lesions deferred revascularization among patients with acute coronary syndrome. J Am Heart Assoc. 2015;4:e002172. doi: 10.1161/JAHA.115.002172 [DOI] [PMC free article] [PubMed] [Google Scholar]


