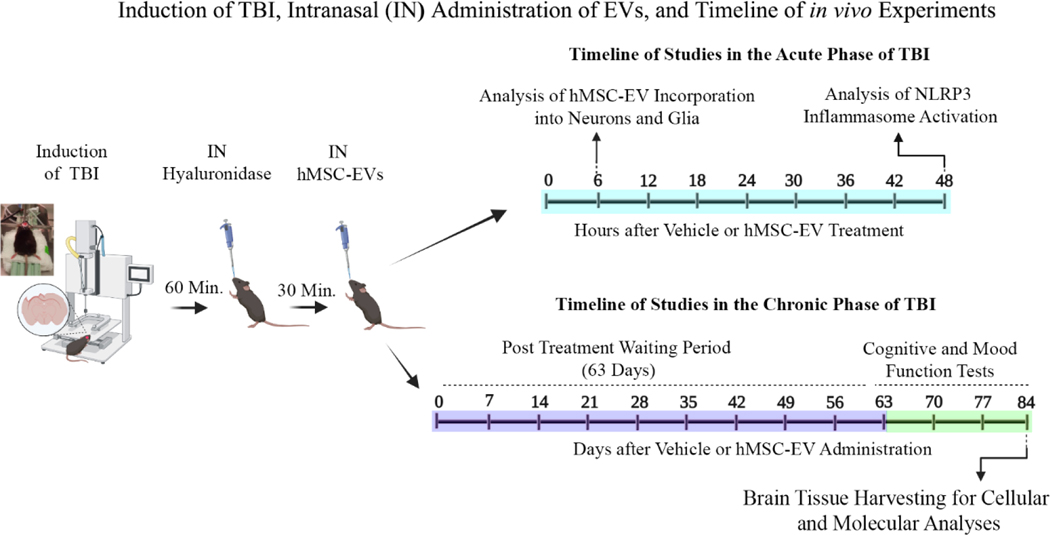
Figure 1: A schematic displaying the various in vivo experiments and analyses performed in the study.
The sequence comprises the induction of controlled cortical impact injury (CCI), intranasal (IN) administration of hyaluronidase at 60 minutes post-TBI, IN administration of hMSC-EVs at 90 minutes post-TBI, and the timeline of studies in the acute and chronic phases of TBI. The experiments in the acute phase of TBI include analyses of hMSC-EV incorporation into neurons and glia at 6 hours post-EV administration and NLRP3 inflammasome activation in the injured brain at 48 hours after vehicle or hMSC-EV administration. The experiments in the chronic phase of TBI comprise cognitive and mood function tests at 63–83 days post-TBI and brain tissue harvesting at 84 days after vehicle or hMSC-EV administration.