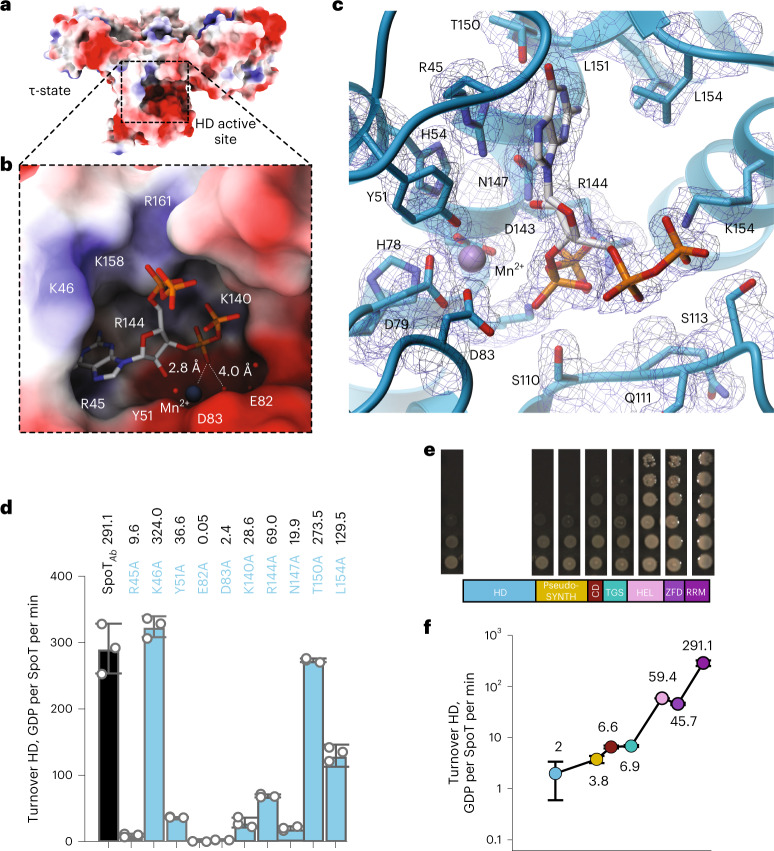
Fig. 3. Defining features of A. baumannii SpoT catalysis.
a, Surface representation of SpoTAb in the τ-state. The active site cavity in the HD domain is boxed in dashed lines. b, Zoom into the HD-active site of the SpoTAb–ppGpp complex. The acidic half of the interface (residues R45, Y51, E82, D83 and K140) and the Mn2+ ion activate the water molecule for nucleophilic attack of the pyrophosphate bond pf ppGpp, while the basic half of the interface (K46, K158 and R161) stabilizes the 3′ and 5′ phosphates of the alarmone substrate. c, Ribbon representation of the active site of SpoTAb revealing the residues involved in coordination of ppGpp. The unbiased mFo-DFc electron density map corresponding to these residues is shown in dark blue. d, Effects of Ala substitutions in the ppGpp binding site on the HD activity of SpoTAb. The residues for substitution were selected as per c. e, The HD functionality test of truncated versions of SpoTAb. SpoTAb variants (each labeled on the figure) were coexpressed expressed with RelAAb in ∆relA∆spoT Ptac::relA A. baumannii (AB5075). The ability of SpoTAb to promote the growth is reflective of its HD competence. f, HD activity of SpoTAb and the C-terminally truncated SpoTAb variants. Turnovers corresponding to each protein variant are colored as per the domain color code in e. Error bars represent s.d. of three or more independent samples examined over three independent experiments.