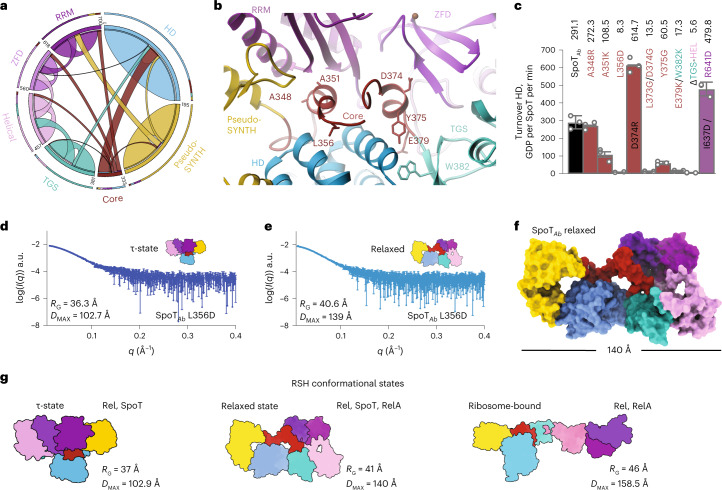
Fig. 4. The CTD controls the hydrolysis activity of SpoT by controlling the equilibrium between HD-active τ-state and HD-inactive relaxed conformations.
a, Circos plot of long-distance interactions (ten residues or more) within SpoTAb. Each domain is defined and colored as in Fig. 2a. The thickness of connecting lines represent the number of contacts between two domains. b, Cartoon representation of the allosteric network defined by the Core domain connecting the domains of the enzyme in the τ-state. The key interface residues are shown as sticks and labeled. c, HD activity of crucial Core residues involved in interactions with other domains of SpoTAb (A384R contacting SYNTH, A351K contacting RRM, L356D contacting HD, L373G/D374G contacting ZFD, Y375G contacting the TGS). The TGS:HD interface is also probed with the E379K/W382K point mutant and ΔTGS–HEL versions. The τ-state stabilizing substitutions D374R and I637D/R641D increase the HD activity. d,e, SAXS curves of L356D in the τ-state (d) or relaxed state (e). f, Pseudo-atomic model of the relaxed state of SpoTAb calculated with Dadimodo49 using the experimental SAXS data from e. g, Cartoon representation of experimentally observed conformational states as well as particle dimensions of long-RSH enzymes. Error bars represent s.d. of three or more independent samples examined over three independent experiments.