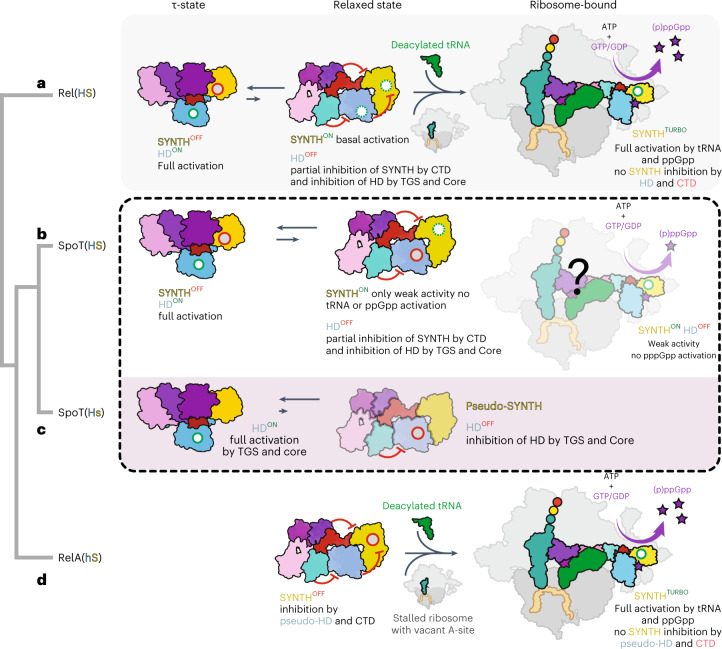
Fig. 6. The enzymatic output of subfunctionalized RelA and SpoT RSH enzymes is evolutionarily tuned through constrains of the conformational landscape.
a, Off ribosomes the ancestral bifunctional Rel[HS] assumes a τ-state with the CTD organizing the HD-active site and promoting the HD activity. This, in turn, strongly inhibits SYNTH activity via intra-NTD regulation. On amino acid starvation, Rel is recruited to starved ribosomal complexes. The ribosome-bound Rel assumes an extended conformation in which the auto-inhibitory effect of the CTD region on the SYNTH activity is released. The full activation of SYNTH is achieved on binding (p)ppGpp to an allosteric site within the NTD releasing the SYNTH inhibition by the HD domain. Thus, the full activation of either SYNTH or HD requires allosteric signaling from CTD to NTD enzymatic domains. b, Evolution of SpoT as a predominantly HD involved the loss of the allosteric control of the NTD by (p)ppGpp. In the bifunctional SpoT[HS] present in most Gamma- and Betaproteobacteria, the enzyme is capable of inefficient (p)ppGpp synthesis in the relaxed state despite the equilibrium strongly favoring the HD-active τ-state; it remains to be determined whether or not synthesis occurs on the ribosome. c, Subfunctionalization of SpoT in Moraxellaceae resulted in the monofunctional HD SpoT[Hs], which naturally populates only the compact τ-state, it is not control by pppGpp or starved ribosomes and is SYNTH-inactive. d, Subfunctionalization of Beta- and Gammaproteobacterial RelA[hS] constitutes the other extreme case of evolutionary restriction of the conformational dynamics of the ancestral Rel[HS]. While losing its HD activity, RelA retains all the allosteric elements of Rel involved in the regulation of (p)ppGpp synthesis and off the ribosome it does not assume the τ-state. Instead, it predominantly populates the functionally frustrated relaxed conformation, primed to switch to the elongated ribosome-associated state triggered by the 70S ribosome, uncharged tRNA and alarmones during stringency. Red circles represent inhibited catalytic centers, green circles represent fully activated catalytic centers and dashed green circles represent idling catalytic centers.